What is catalytic activated carbon?
Activated carbon has developed pore structure, large specific surface area and good strength, and is widely used as an adsorbent for adsorption removal, purification or recycling of a certain component or certain components in liquid or gas;
On the other hand, activated carbon also has the advantages of economy, greenness, and easy to change the chemical properties of the surface, etc., and therefore, like molecular sieve and alumina, it has been used as catalysts and catalyst carriers.
The use of chlorine gas by the German army during the First World War (1915) led to worldwide research into the use of activated catalytic carbon in filter of gas masks.
During this time, it was discovered that the decomposition of toxic gases by activated carbon could be accelerated by impregnation with metal salts, thus initiating research on activated carbon as a catalyst carrier.
The catalytic activity of activated carbon when used as a contact catalyst is due to the surface and surface compounds of the carbon as well as ash, etc. It is mainly used in various isomerization, polymerization, oxidation and halogenation reactions.
Activated carbon is often used as a catalyst carrier in the chemical industry, that is, substances with catalytic activity are deposited on activated carbon and used together as a catalyst.
At this time, the role of activated carbon is not limited to loading activator, it has the role of co-catalyst, and has a significant impact on the activity, selectivity and service life of the catalyst. People usually use columnar activated carbon, granular activated carbon, and honeycomb activated carbon as carriers to produce activated catalytic carbon. This paper provides an introduction to the application of activated carbon as a catalyst and catalyst carrier.
1. Catalytic effects of activated carbon
Activated carbon has the ability to catalyze a wide range of reactions, and practice and research have shown that the catalytic activity of many metals and metal oxides is due to the presence of active centers, which are mostly crystalline defects.
The microcrystals in activated carbon (especially along the edges of the crystal lattice) consist of a large number of unsaturated valence bonds that have a structure similar to crystalline defects, which gives the activated carbon its catalytic activity.
If the action of activated catalytic carbon catalysis is explained from the point of action, it can be roughly categorized into the actions of electron-conducting and electron-conducting based surface radicals, and surface oxide functional groups (including acidic, neutral and basic functional groups).
The presence of these functional groups also has an important influence on the catalytic properties of activated carbon.
1. Modification of activated carbon as catalyst and catalyst carrier
The modification methods of activated carbon generally use high temperature heat treatment, oxidation treatment (including gas-phase oxidation and liquid-phase oxidation), ultrasonic treatment, microwave treatment, hydrothermal treatment, and electrochemical method.
(1) High-temperature heat treatment
Heat treatment of activated carbon under the protection of isolated air or inert gas is an important method of activated carbon modification.
At high temperatures, the graphitization of the activated carbon increases, the surface heteroatom groups will be cleaved, and the hydrophobicity and alkalinity of the activated carbon increase.
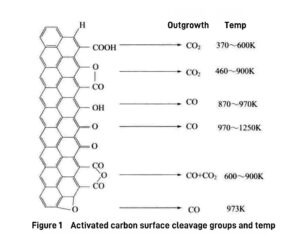
Generally, the decomposition temperature of carboxylic acid groups on the surface of activated carbon is low, while carbon dioxide is released, while the decomposition temperature of phenolic hydroxyl, ether and carbonyl groups is high, while carbon monoxide is released.
Figure 1 shows the groups released during the decomposition of activated carbon surface groups and the corresponding decomposition temperatures.
(2) Oxidative treatment
Oxidative treatment is a commonly used treatment for modification of activated carbon, and its main purpose is to introduce oxygen-containing groups on the surface.
Oxidation treatment mainly includes liquid-phase oxidation and gas-phase oxidation, and most of the commonly used liquid-phase oxidizing agents reported in the literature include nitric acid, hydrogen peroxide, potassium permanganate, sulfuric acid, mixed acid of nitric acid and sulfuric acid, ammonium persulfate and sodium hypochlorite; gas-phase oxidizing agents include O₂, O₃, O₂/N₂ mixture, N₂O, CO₂, NO₂, and air.
However, when activated carbon is used as catalyst carrier, especially for Ru/Ac ammonia synthesis catalyst, due to the strong toxic effect of elements such as S, CI, etc. on the catalyst, ammonium persulfate, sodium hypochlorite and sulfuric acid are not suitable oxidizing agents.
(3) Other treatments
The activity of the ruthenium-based ammonia synthesis catalyst loaded on ultrasound-modified activated carbon was greatly improved by ultrasound treatment of the carrier activated carbon compared with the conventional treatment.
The microwave treatment of activated carbon under nitrogen protection for 5 min also significantly increased the ammonia synthesis activity of the catalyst without significant changes in the specific surface area and pore volume of the carrier.
2.Application of activated carbon as a catalyst
Table: Main application areas of activated carbon as a catalyst
| Type of reaction | Specific types of reactions |
|---|---|
| Halogen-containing reactions | The reaction of producing phosgene from carbon monoxide, the reaction of producing uric acid chloride, the reaction of producing trichloroethylene and tetrachloroethylene, the fluorination reaction, the reaction of producing chlorosulfonic acid and fluorosulfonic acid, the chlorination reaction of ethanol; the chlorination reaction of producing ethylene dichloride from ethylene |
| Oxidation reaction | Oxidation of sodium sulfide in aqueous solution to produce polysulfide compounds; Oxidation of sulfuric acid from sulfur dioxide gas; Oxidation of elemental sulfur from hydrogen sulfide; Oxidation of nitric oxide; Oxidation of oxalic acid; Oxidation of ethanol |
| Dehydrogenation reaction | Dehydrogenation of alkenes from alkanes; Dehydrogenation of aromatic compounds from cycloalkanes |
| Oxidation and dehydrogenation reactions | Oxidation and dehydrogenation reactions of alkanes to alkenes |
| Reduction reaction | Reduction of olefins and dienes; Reaction to produce methanol by reduction of carbonyl groups; Hydrogenation of fats and oils; Reduction of aromatic carboxylic acids; Decomposition of peroxides; Reduction of nitric oxide to ammonia |
| Monomer synthesis reaction | Synthesis reaction of vinyl chloride monomer; Synthesis reaction of vinyl acetate monomer |
| Isomerization reaction | Isomerization reaction of butadiene; Isomerization reaction of cresol; Isomerization reaction of rosin and oils, etc. |
| Polymerization | Polymerization of ethylene, propylene, butylene, styrene, etc. |
| Other reactions | Dehydration reaction of alcohols, deuterium exchange reaction |
The role of activated carbon in flue gas desulphurization (FGD)
Since activated catalytic carbon has good SO₂ adsorption properties and can catalytically oxidize SO₂ to H₂SO₄ in the presence of O₂ and H₂O, a flue gas desulphurization (FGD) method that utilizes activated carbon for the adsorption of SO₂ has been studied and developed.
Activated carbon method of flue gas desulfurization is a process of physical adsorption and chemical adsorption at the same time, there are a series of chemical reactions on the surface of activated carbon, but due to the complexity of the surface condition of activated carbon, the adsorption mechanism is divided into different opinions.
For a long time, there are many kinds of claims of activated carbon desulfurization mechanism, accounting for the mainstream viewpoints there are two kinds, one of them is: activated carbon can remove SO₂ in the presence of O₂ and a small amount of water vapor.
After SO₂ is adsorbed on the surface, it is catalytically oxidized to SO₃, which then interacts with water to form H₂SO₄. In the presence of excess water, H₂SO₄, is removed from the surface, thus vacating the active site for SO₂ adsorption, so that the cyclic process of adsorption, oxidation, hydration of SO₂, and generation and desorption of H₂SO₄, is carried out continuously.
The total course of the reaction can be described by the following chemical equation:
SO₂+H₂O+O₂ → H₂SO₄:
3.Preparation and application of loaded activated carbon
The use of activated carbon as a carrier has a much wider range of applications than activated carbon itself as a catalyst.
Activated carbon as a carrier is not as limited as alumina, and can be loaded with noble metals (e.g., Pt, Pd, Pu, Ph, Re, Os, Ir, etc.), sulfides (e.g., MnS, MoS2, WS2, HgS, ZnS, CuS, CdS), halides (AlCl₃, alkaline-earth metals, chlorides, etc.), and inorganic acids, which can be used for hydrogenation or synthesis of pesticides, pharmaceuticals and spices, production of polyesters and lipocyclic compounds in plastics and chemical fibers, and polycarbamates in lipids and fats.
It is mainly used in the hydrogenation or synthesis of pesticides, pharmaceuticals and spices, the production of polyester and polyurethane in plastics and chemical fibers, and the dehydrogenation of alicyclic and alicyclic compounds to make aryclic compounds.
One of the more popular applications is the use of activated carbon loaded with rare precious metals, since one of the advantages of using activated carbon loaded with precious metals is the easy recycling of the precious metals, e.g., by heating and combusting the used catalyst for disposal.
The general process of using activated carbon as a carrier is to load the metal salt impregnation onto the activated carbon first, and then the activated carbon containing precious metals is heated, such as the loading of Pt is to load the platinum acid salt first, and then undergo pyrolytic decomposition treatment, the resulting Pt is loaded onto the activated carbon with tiny particles, but for other transition metal salts, such as iron salts, high-temperature heat treatment is obtained after the corresponding metal oxides.
In catalytic environments with high acid-base intensity, such as the reduction reaction of nitrobenzene, the alumina and silicon oxide molecular sieve carriers will not be able to withstand such environments, whereas activated carbon carriers do not have such problems.
Moreover, activated carbon has better activity than SiO₂, Al₂O₃, molecular sieves and polymer carriers in reactions such as the carbonyl synthesis of acetic acid from methanol and the carbonyl synthesis of propionic acid from ethanol.
1. The role of activated carbon as a catalyst carrier
Carrier is the dispersant, binder and support for the main catalyst and co-catalyst in the solid catalyst. The role of carriers can be summarized as follows.
(1)Diffusioneffects
Multiphase catalysis is an interfacial phenomenon, so the active components of catalysts are required to have sufficient surface area, which needs to improve the dispersion of the active components so that they are in micrometer or atomic level dispersion.
And the carrier can disperse the active component into very small particles and keep its stability. For example, when the noble metal Pt is loaded on Al₂O₃ carrier, Pt is dispersed into nanoscale particles and becomes a highly active catalyst, which in turn greatly improves the utilization rate of the noble metal.
(2)Stabilizing effects
The carrier can play a stabilizing role for the catalyst by preventing the microcrystals of the active component from semi-melting or recrystallizing.
The carrier can block the microcrystals and prevent them from migrating under high temperature conditions.
For example, hydrocarbon steam reforming to hydrogen catalyst, when aluminum magnesium spinel is chosen as the carrier, it can prevent the microcrystalline grains of the active component Ni from growing up at high temperature (1073K).
(3)Supporting roles
The carrier can give the solid catalyst a certain shape and size, so that it meets the requirements of industrial reaction on the hydrodynamic conditions of the catalyst.
The carrier can also give the catalyst a certain mechanical strength, so that it will not be broken or pulverized in the process of use, in order to avoid the increase of catalyst bed resistance, so as to make the fluid distribution uniform and keep the process operation conditions stable.
(4)Heat transfer and dilution effects
For strongly exothermic or strongly heat-absorbing reactions, by choosing catalyst carriers with good thermal conductivity, the heat of the reaction can be removed in time, thus preventing the surface temperature of the catalyst from being too high.
For highly active components, adding appropriate amount of carrier can play the role of dilution and reduce the activity per unit volume of catalyst to ensure the thermal balance.
Both roles of the carrier can make the reaction temperature of the catalyst bed constant, and at the same time make the thermal stability of the active components improved.
(5)Catalytic role
In addition to the physical effects described above, carriers also have chemical effects. The activity, selectivity and stability of the catalyst may change due to the chemical interaction between the carrier and the active component or co-catalyst.
In highly dispersed loaded catalysts, co-catalytic effects may occur due to the strong interaction or induced effect of the oxide carriers on the metal atoms or ionic active components.
On the other hand, the acid-base nature of the carrier may also have a multifunctional catalytic effect with the metal active component, making the carrier part of the active component and forming a bifunctional catalyst.
2.Conditions of activated carbon as catalyst carrier
| category | reaction | Active ingredients |
|---|---|---|
| Monomer manufacturing | Synthesis of Vinyl Chloride from Vinyl Acetate | Zinc acetate mercuric chloride, zinc acetate with alkali metal and alkaline earth metal chloride as promoter |
| Halogenation and dehalogenation reactions | Cyanuric chloride is used to produce hydrochloric acid and hydrobromic acid. It is used to synthesize freons. It is used to produce trichloroethylene. It is used to synthesize hexachlorobenzene. It is used to synthesize hydrocarbons. It is used to produce chlorinated alcohols. | Metal halides Ferric chloride, Cupric chloride, Chromium chloride Metal halides Ferrous chloride, Barium chloride Aluminum chloride Metal chlorides Phosphoric acid, Calcium chloride, Zinc chloride |
| oxygenchange | Oxidation of alcohols Oxidation of olefins Oxidation of p-isopropylbenzylmethane Oxidation of steroids Oxidation of ethylene to acetaldehyde | Platinum, palladium, copper, silver nitrate, silver oxide, platinum, palladium, palladium, titanium, palladium, vanadium, chromium, molybdenum, silver |
| reduction | Carboxylic acid reduction Unsaturated acid reduction Olefin reduction Nitro and nitroso compound reduction Pyridine derivative reduction Carbazole reduction | Ruthenium Nickel Nickel, Cobalt Rhodium Rhodium, Ruthenium |
| Isomerization | Formic acid isomerization Rosin isomerization Vegetable oil isomerization Olefin isomerization Hydrocarbon isomerization | Zinc chloride phosphate, palladium nickel platinum phosphate |
| Hydration | Acetylene hydration Ethylene hydration | Sulphides or phosphates of mercury, zinc, copper, cadmium, manganese, thorium oxide, phosphoric acid, phosphates, sulphuric acid, magnesium oxide, iron, potassium carbonate |
| Dehydrogenation | Dehydrogenation of alkanes and cycloalkanes Dehydrogenation of hydrocarbons | Platinum, nickel ① sodium salt, lithium salt; ② nickel |
| polymerization | Ethylene polymerization Propylene polymerization Olefin polymerization Butadiene polymerization | Cobalt, nickel, nickel oxide, alkali metal solid titanium phosphate, zirconium, cobalt, nickel, phosphoric acid, nickel cobalt oxide, nickel |
| Hydrocracking | Tar hydrocracking Oil hydrocracking | Oxides and sulfides of molybdenum and tungsten Sulfides of molybdenum and tungsten |
| Other reactions | Synthesis of aldehydes and alcohols from carbonyl compounds. Production of acetic acid. Synthesis of carbon disulfide. Synthesis of olefins. Synthesis of esters. Synthesis of acrylonitrile. Production of benzene. Amination of alkylated alcohols. Production of tetrahydrofuran derivatives. Condensation, polymerization, alkylation of hydrocarbons. Production of acrolein. Production of methyl vinyl ether. Production of alcohols from ethers. | Chromium, nickel, iron, manganese, mercury, cobalt, platinum, ruthenium, molybdenum sulfide, phosphoric acid, zinc oxide, iron, cobalt, aluminum oxide, silicate, alkali, carbonates of alkaline earth metals, cyanide, zinc chloride, boron chloride, platinum phosphate, sodium carbonate, potassium carbonate, 50% potassium hydroxide, phosphoric acid |
3. Preparation method of loaded activated carbon
The main methods for preparing loaded catalysts using activated carbon as carrier are: impregnation, chemical co-precipitation and ion exchange.
(1)Impregnation method
The impregnation method is commonly used to prepare loaded metal catalysts, especially for noble metal catalysts, which can realize the uniform distribution of metal on the carrier with low loading capacity.
At the same time, the carrier can also improve the heat transfer of the catalyst and prevent the sintering of the metal particles, etc. After the soluble compound of Pt is dissolved, it is mixed with the carrier, and then a reducing agent, such as NaBH4, formaldehyde solution, sodium citrate, sodium formate, or hydrazine, is added to make the Pt reduced and adsorbed on the carrier, and then it is dried to produce Pt/C catalyst.
Brown’s method with NaBH4 as the reducing agent and Kaffer’s method with hydrazine as the reducing agent are the most representative reactions for the preparation of loaded metal catalysts by impregnation.
The advantages of this method are that it is done in a single step, the process is simple and can be used for the preparation of electrocatalysts ranging from mono- to polycatalysts; and it can be operated in the aqueous phase.
learn more:
(2) Chemical coprecipitation
Co-precipitation is also a common method for the preparation of loaded metal catalysts.
Watanabe et al. used the co-precipitation method for the preparation of Pt-Ru/C catalysts, which is characterized by the use of hydrogen peroxide to oxidize the platinum and ruthenium metal salts.
A sol of PtO₂ and RuO₂ is formed, which is then routed with carbon black, and the oxides are reduced in the liquid phase by the injection of hydrogen, resulting in a stretcher-loaded Pt-Ru alloy.
The main advantages of this process are that highly dispersed Pt/C and Pt-Ru/C electrocatalysts can be prepared at high metal loading [for 10% (mass fraction) Pt/C, the size of metal particles is about 2 nm]; chlorine-containing noble metal salt precursors can be utilized to prepare Pt/C and Pt-Ru/C catalysts that are virtually free of chlorine ions; and the operation can be carried out in aqueous phases in an environmentally friendly manner.
(3) Ion exchange
The surface of carbon carriers contains various types of structural defects in varying degrees, and functional groups such as hydroxyl and phenol groups can easily combine with the carbon atoms at the defects, and these surface groups can be exchanged with ions in the solution, which is the principle used to prepare highly dispersible catalysts.
The ion exchange method utilizes this principle to prepare highly dispersible catalysts. Gamez et al. used different pretreatment methods, such as treating carbon with oxidizing agents such as HNO₃ and NaClO, to increase the surface groups of carbon, then exchanging ions with Pt(NH3)4(OH)2, and then reducing adsorbed Pt(NH3)4(OH)2 with hydrogen to obtain nano-sized catalysts, but the nanoscale catalysts produced by this method were not as good as those produced by the ion exchange method.
However, the amount of Pt on the carbon carrier in the catalyst produced by this method is limited by the exchange capacity of the carrier.
(4) Gas phase reduction
The compounds of Pt were impregnated or precipitated on activated carbon, which was later dried and reduced by H₂ at high temperature to obtain the catalysts. Alersool et al. loaded Pt(NH3)4(NO3)2 and RuRu(NH3)6Cl3 onto SiO₂ and prepared Pt-Ru/SiO₂ catalysts with particle sizes ranging from 2.5 to 3.0 nm by H₂ reduction for 4h at 400 °C, but when first heat-treated in O₂ atmosphere for 1h and then reduced by H₂ under the same conditions, metal particles with sizes of 1.0~1.5 nm were obtained.
(5) Electrochemical methods
Pt or other metals can be reduced using electrochemical methods such as cyclic voltammetry, constant potential, underpotential deposition, and square wave scanning techniques.
Morimoto et al. prepared Pt, Pt-Ru, and Pt-Sn catalysts using the constant potential technique and compared their catalytic oxidation behaviors of CO, and found that compared to Pt, the Pt-Ru, and Pt-Sn catalysts had better anti-toxic chemical Ren et al. used cyclic voltammetry and square wave scanning techniques to fabricate Pt electrodes with different roughness and investigated their catalytic behavior for methanol oxidation using Raman spectroscopy.
Massong et al. deposited Sn and Bi on different crystalline surfaces of Pt by underpotential (UPD) deposition technique, and by studying the electrocatalytic oxidation of CO, it was found that Sn deposited on the crystalline surface of Pt(111) had a lower hydrogen adsorption behavior has a low hydrogen adsorption behavior.
However, for the preparation of loaded metal catalysts by electrochemical methods, how to load the metal catalysts onto the activated carbon uniformly and the control of the metal content of each component during the co-deposition process is a more difficult problem to control.
(6) High-temperature alloying method
High performance catalysts are obtained by alloying polymetals with high temperature technology. Ley et al. produced a Pt-Ru-Os ternary alloy by argon arc melting (Arc-melt) technique, which is beneficial to reduce the CO coverage on the surface of Pt, and thus exhibits excellent electrocatalytic performance.
The electrocatalytic oxidation current density of methanol on this catalyst can reach 340mA/cm2 at 90 C and 0.4 V, while that of the common Pt-Ru catalyst is only 260mA/cm2.
In addition, Kabbabit et al. used a high-frequency furnace with a magnetic levitation device to produce Pt-Ru alloys with different atomic ratios.
(7) Sol-gel method
The sol-gel method is an effective method for the preparation of nanosized catalyst particles.
The most typical one is Bonnemann’s method, which is a method that uses N(C4H17)4BEt3H to react with a metal salt solution in an organic solvent to generate a metal sol, where N(C4H17)4 acts as a stabilizer for the sol, and BEt3H- acts as a reducing agent.
Carbon black was added to the sol, followed by filtration, washing, and drying to obtain carbon-loaded catalysts with an average particle size of about 2.1 nm.
Schmidt et al. used this method to produce Pt-Ru/C and Pd-Au/C catalysts, respectively, which were comparable to the commercial E-TEK catalysts of the same type.Gotz also prepared Pt-Ru, Pd-Au, and Pd-Au catalysts in this way.
Gotz also prepared a series of colloidal metal catalysts with a particle size of 1.7 nm, such as Pt-Ru, Pt-Ru-Sn, Pt-Ru-Mo, and Pt-Ru-W, by this method.
(8) Other methods
The method of microwave heating has also been used for the rapid preparation of Pt-Ru/C anode electrocatalysts.
In this method, a Pt-Ru homologous molecule was used as a precursor of Pt-Ru metal, which was heated to decompose in a microwave field after adding a carrier, and then a hydrogen-nitrogen mixture was introduced into the microwave field as a reducing agent.
Although the process of using microwave heating is faster, the preparation process of its Pt-Ru homologous molecule is very complicated.
Moreover, the reduction process is carried out in the microwave field, the reaction temperature is high and difficult to control, which is dangerous.
It is worth mentioning that combinatorial chemistry techniques have also been applied to the screening and preparation of chemical components for fuel cell electrocatalysts.
Choi et al. cleverly used an ordinary color printer to combine H2PtCl6・XH2O, RuCl3 , (NH4)6W12O39・XH2O , MoCl5, respectively, into four different cartridges, and then the four precious metals were continuously printed onto carbon paper with different contents, using NaBH4, and the electrode was used as a working electrode after reduction, and the potential step scanning was carried out in methanol with added indicator, and the results showed that, at a low overpotential, the highly active electrocatalysts in the region of methanol reacted first to produce hydrogen protons, and the catalyst was screened by the fluorescence emitted by the indicator in the acidic medium.
4. Application technology of loaded activated carbon catalysts
Activated carbon loaded metal catalysts and their applications
The main metals loaded on activated catalytic carbon are vanadium (V), manganese (Mn), copper (Cu), iron (Fe), cobalt (Co), nickel (Ni), platinum (Pt), titanium (Ti) and so on, and so far, there have been a lot of different kinds of metal catalysts on activated carbon carriers that have realized industrial applications.
(1) Activated carbon loaded iron catalyst and its application
Iron has been widely studied as the most common metal in daily life and is cheap and easy to obtain, and has been applied in the field of flue gas desulfurization (FGD).
It was shown that when AC was loaded with iron before carbonization, the number of basic groups on the surface of AC increased significantly with the increase of iron content, while the number of acidic functional groups decreased slightly; whereas, for the material loaded with iron after activation, the number of acidic and basic functional groups on the surface did not change significantly.
At low temperature (373 K), the adsorption capacity of SO₂ increases with the increase of iron content, and, the good dispersion of iron was beneficial to improve the adsorption oxidation of SO₂ by the materials.
It has been confirmed that the adsorption capacity of the material for SO, increases with the simultaneous presence of NOx, in the reaction gas.
Ma et al. investigated the desulfurization performance of Fe/AC at 423~523 K, and showed that in the experimental temperature range, the removal capacity of the material for SO₂ increased with the temperature.
At 473 K, the adsorption capacity of Fe/AC for SO₂ was much higher than that of AC or Fe2O3 alone.
The programmed warming desorption experiments revealed that after the material went through the desulfurization process, its surface generated H₂SO₄. andFe2(SO4)3.
The AC-based desulfurization catalysts with surface-loaded iron oxides were more regenerable, the iron oxides were not easily reduced during the regeneration process, and the Fe/AC had a relatively long lifetime.
(2) Activated carbon loaded Cu catalysts and their applications
The study of CuO catalysts loaded on activated carbon is also extensive. Tseng et al. compared the performance of CuO, Fe2O3 and V2O5 loaded on AC for the simultaneous removal of SO₂, NO, and HCI from flue gas under the same experimental conditions.
The results showed that the catalytic process of SO₂ on metal oxides consists of three stages: SO₂ adsorption to form sulfite, sulfite oxidation to form sulfate, and sulfate decomposition to release SO₂.
Moreover, it was found that CuO/AC only acted as an adsorbent and that copper oxide deactivated the catalyst during the catalytic process because of copper sulfate.
Gao Jingjing prepared a series of adsorption desulfurizers using activated carbon as a carrier and CuO, ZnO and nitric acid as modifiers by equal volume impregnation method, and evaluated the adsorption of this series of desulfurizers in diesel adsorption desulfurization by static adsorption and fixed bed dynamic adsorption methods.
The results showed that the activated carbon-based adsorbents were effective in removing the sulfur-containing compounds in diesel fuel.
The adsorption desulfurization performance of modified activated carbon was better than that of activated carbon as is (AC), the desulfurization performance of activated carbon loaded with CuO (CuO/AC) was better than that of activated carbon loaded with ZnO (ZnO/AC), and the desulfurization performance of activated carbon mixed loaded with CuO/ZnO (CuO-ZnO/AC) did not differ much from that of activated carbon loaded with either CuO or ZnO alone.
The desulfurization performance of activated carbon activated by nitric acid and then loaded with CuO (AC-N-A) was greatly improved, and its desulfurization performance was better than that of direct loading of CuO.
Activated carbon loaded with CuO and ZnO is mainly used for the removal of dibenzothiophene and its derivatives from diesel fuel.
Through the regeneration study of desulfurizer AC-N-A, it was found that the use of gas blowing thermal regeneration and organic solvent elution regeneration had a certain regeneration effect on the deactivated desulfurizer.
(3) Activated carbon loaded Ti catalysts and their applications
TiO₂ has the photocatalytic performance of reducing energy consumption and saving energy, so its study has received more and more attention and become a hot research topic in recent years.
At present, the research on TiO₂ basically focuses on photocatalysis, while not much research has been done on the thermodynamic reactions in which it is involved.
In terms of environmental protection, the research on TiO₂ is dominated by wastewater treatment, using it in water filtration. Since the photocatalytic function of TiO₂ is not selective, it is suitable for the treatment of many kinds of wastewater such as phenol-containing wastewater and dye wastewater.
With the deepening of research in this area, TiO₂ is expected to be industrialized in the field of wastewater treatment. In the treatment of gaseous pollutants, a study found that TiO₂ in the treatment of indoor formaldehyde air pollution shows broad application prospects, the application is worth in-depth study, there are related products on the market.
In flue gas desulfurization, there have been domestic and foreign reports on the use of composite materials of titanium dioxide and activated carbon as desulfurization agents, and there has been a boom in research on the preparation technology of TiO₂ and AC composite materials.
(4) Activated carbon loaded Pt catalysts and their applications
① Application in hydrogenation-dehydrogenation.
In recent years, with the development of hydrogen energy economy, the methylcyclohexane (MCH) dehydrogenation reaction has gradually gained wide international attention as one of the commonly used probe reactions.
This method is used to store and transport hydrogen efficiently through a hydrogenation-dehydrogenation cycle. In this reaction, Pt catalysts with carbon materials as carriers exhibit a number of different properties.
Xiaoyun Li et al. from the State Key Laboratory of Fundamentals of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, treated activated carbon by different methods and prepared activated carbon loaded Pt catalysts by conventional impregnation method, and investigated their catalytic performances in the dehydrogenation reaction of methylcyclohexane, respectively.
Through the characterization of nitrogen adsorption and programmed elevated temperature desorption of the carbon carriers, the results showed that the pore structure of the activated carbon was basically unchanged after oxidative treatment with nitric acid and high-temperature treatment with hydrogen, but the number and types of oxygen-containing functional groups on the surface changed, and the dispersion of the Pt particles on the carriers was directly affected by the surface groups, which then caused the catalysts to exhibit different activities in the reaction.
② Application in methanol fuel cell.
Direct methanol fuel cell (DMFC), is a fuel cell with ion exchange membrane as electrolyte, methanol as anode fuel and air as oxidizer.
Compared with gaseous fuel, methanol has the advantages of easy reserve and transportation, high energy conversion efficiency, no pollution of reaction products, etc.
Therefore, direct methanol fuel cell is an environmentally friendly green energy source. DMFC is regarded as one of the most promising portable power sources, which has the outstanding advantages of small size, light weight, high efficiency, etc., and has shown broad application prospects and huge potential market in the fields of transportation, communication and aerospace.
At present, the most used cathode electrocatalyst for DMFC is Pt/C catalyst, which shows high catalytic activity for redox reactions in both acidic and alkaline media.
Despite the high catalytic activity and stability of Pt for redox reactions, it is expensive and resource-limited, and further reduction of Pt loading is necessary for the commercialization of fuel cells.
It has been found that Pt-based alloy catalysts have higher catalytic activity compared to monomeric Pt catalysts. Various Pt-based alloys have been used as electrocatalysts for redox reactions over the past two decades.
(5) Activated carbon loaded Co catalysts and their applications
Currently, Co-based catalysts are considered to be the most promising catalysts for F-T synthesis because of their strong chain growth ability in F-T synthesis reaction, as well as stable performance, low carbon accumulation, and almost no water gas shift reaction.
In recent years, the Institute of Large Chemical Physics of the Chinese Academy of Sciences (ILCS) has developed a Co/activated carbon catalyst using a fixed-bed process, which is a characteristic Co-based catalyst for the one-step preparation of liquid fuels via Fischer-Tropsch synthesis.
The catalyst can synthesize naphtha and diesel fractions with high selectivity, and the resulting products are basically free of wax generation, and the catalyst is easily separated from the products.
(6) Activated carbon loaded V catalysts and their applications
Vanadium is the most commonly used and most studied catalyst for flue gas desulfurization and denitrogenation.
Usually the oxidation form of vanadium catalysts on AC is V2O5. Many studies have shown that when SO₂ and H₂O are present at the same time, some of the pores of the catalysts will be blocked by the deposited ammonium sulfate salts and cause deactivation.
However, when there is no H₂O present above 180°C, the catalysts are able to promote the reaction between the ammonium sulfate salts and NO, and, the formation of sulfate can make the surface of the catalysts produce new acidic sites and thus improve the adsorption capacity of NH3, therefore, the presence of SO₂ at this time is not only harmless, but on the contrary, it can further improve the denitrogenation performance of the V2O5/activated carbon catalyst.
The simultaneous removal of SO₂ and NO using V2O5-loaded honeycomb activated carbon catalyst system was investigated and compared with catalytic granular activated carbon system catalyst and powdered activated carbon system catalyst.
It was shown that: The removal of SO₂ and NO was better performed with 2% (mass fraction) V2O5 loading on honeycomb activated carbon at about 200 °C, and the selective catalytic reduction was significantly enhanced.
When the catalyst was regenerated by 5% (v/v) NH3/Ar for SO₂ removal, not only the removal rate was slightly increased but also the selective catalytic reduction was more significantly enhanced.
However, microporosity, surface area, SO₂ and NO removal were significantly reduced by the use of binder during honeycomb activated carbon preparation.
Recently, a new type of loaded V2O5 honeycomb AC was prepared by Shanxi Institute of Coal Chemistry, China, and applied to the experimental study of simultaneous desulfurization and denitrogenation.
It was shown that: For SO₂, the adsorption capacity of V2O5/AC was 5.5% when the temperature was 423K or 453K. When the loading content of V2O5 was increased from 1% to 3%, the adsorption capacity of SO₂ would increase from 4.5% to 5.9% accordingly.
This study proves that V2O5/AC can effectively desulfurize and denitrogenate flue gas at low temperature, and provides an effective reference for future industrial practice. Davin compared the effects of vanadium, nickel, cobalt, iron, and manganese metal oxides loaded on AC on SO₂ removal.
The order of catalytic activity of each of these metal oxides was found to be in the following order: vanadium, iron, nickel, drilling, and manganese.
The presence of O₂ in the gas mixture helped to increase the adsorption capacity of the AC by about 10% compared to the unloaded AC with no metal, and the increase in adsorption was even greater compared to the vanadium loaded AC.
In this case, the metal impeded the immobilization of SO₂ on the AC and catalyzed the conversion of SO₂ to other more stable forms (due to the presence of O₂).
When both O₂ and H₂O exists simultaneously, water vapor adsorption on the AC causes SO₂ or its transformation products to dissolve on the AC, which promotes the continued adsorption of SO₂, and therefore, the adsorption capacity of the V2O5/AC is further increased.
It can be concluded that the SO₂ treatment is more effective when metal and moisture are present at the same time, and catalysis and dissolution work together. Another experiment showed that SO₂ conversion was better when the content of vanadium was 0.3% (mass fraction).
Carabineiro et al. investigated the adsorption behavior of AC impregnated with Ba, Co, Cu, Fe, Mg, Mn, Ni, Pb, V and their binary mixtures of SO₂ at 293 K.
It was found that AC impregnated with mixtures of V and Cu showed optimum desulfurization effect, and the two have a synergistic effect.
(7) Activated carbon loaded Mn catalyst and its application
Teresa et al. through the honeycomb activated carbon loaded manganese oxide selective catalytic removal of NOx, the study found that: when the temperature is low (150C), the airspeed of 4,000h-1, the removal rate of NOx can reach 60%-70%, but the sulfur dioxide in the flue gas will react with the loaded manganese to form manganese sulfate to impede the reaction and make the manganese inactive.
In addition, it was found that the catalytic activity increased with the increase of temperature, but the selectivity decreased.
(8) Activated carbon loaded with other metal catalysts and their applications
Research on AC-based desulfurization catalysts for other transition metals (e.g., manganese) and alkali metals or alkaline earth metals (e.g., potassium, calcium) is also in progress.
In view of the fact that conventional flue gas desulfurization (FGD) methods usually use oxides or hydroxides of alkali or alkaline-earth metals as desulfurizers, some researchers have loaded such desulfurizers onto activated carbon in order to take advantage of both conventional desulfurizers and ACs at the same time.
This is similar to soft manganese ore desulfurization, where manganese oxide loading also enables flue gas desulfurization. American Dukangla and EPA studied 48 kinds of metal oxides and found that in the range of boiler flue temperatures, the oxides of Fe, Co, Ni, Cr, Cu, Ce can not only make SO₂ effectively removed, but also the adsorbent itself can be well regenerated, and the rate of adsorption is high, which is of good practicability.
learn more: