Definition of impregnated activated carbon
Impregnated activated carbon meaning: impregnated activated carbon is a modified activated carbon made by coating or impregnating specific chemical reagents on the surface of activated carbon.
What are the classification and application of impregnated activated carbon?
1)Classification by raw materials of impregnated carbon:
- Coal pelletized , wooden pelletized impregnated activated carbon: mainly used for gas purification and protection.
- Coconut shell granular impregnated activated carbon: mainly used for water purification, catalyst carrier catalyst, gas mask and other protective products.
- Wood granular impregnated activated carbon: more used in decolorization, such as copper, magnesium impregnated activated carbon.
2)Classification by impregnant type
(1)Metal impregnated activated carbon
- Silver impregnated activated carbon: used for antibacterial, catalytic oxidation and other applications.
- Copper impregnated activated carbon: used for adsorption of pollutants such as ammonia and cyanide.
- Manganese impregnated activated carbon: used for redox reactions, such as removing iron and manganese.
(2)Acid and alkali impregnated activated carbon
- Alkali impregnated activated carbon such as Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Potassium iodide (KI), Sodium carbonate (Na₂CO₃), Ammonium bicarbonate (NH₄HCO₃): used for adsorption of acidic gases such as sulfur dioxide (SO2) and hydrogen sulfide (H2S).
- Acid impregnated activated carbon (such as phosphoric acid H3PO4, sulfuric acid H2SO4): used to adsorb alkaline gases such as ammonia (NH3).
(3)Oxidant impregnated activated carbon
- Potassium permanganate (KMnO4) impregnated activated carbon: used to adsorb and oxidize organic compounds and sulfides.
- Hydrogen peroxide (H2O2) impregnated activated carbon: used to enhance oxidation capacity.
(4)Composite impregnated activated carbon
- Composite impregnation of multiple chemical reagents: combined with the advantages of multiple impregnating agents, used for the removal of complex pollutants. For example, composite impregnated activated carbon with potassium iodide (KI) and sodium hydroxide (NaOH) can be used to adsorb and remove mercury vapor.
3)Classification by use
(1)Impregnated activated carbon for air purification
- Antibacterial type: impregnated with metal ions such as silver and copper, used in air purifiers and masks.
- Desulfurization type: impregnated with alkaline compounds, used to remove hydrogen sulfide (H2S) in industrial exhaust gas.
(2)Impregnated activated carbon for water treatment
- Heavy metal removal type: impregnated with sulfide or sulfate, used to adsorb heavy metals such as lead (Pb) and mercury (Hg) in water.
- Residual chlorine removal type: impregnated with reducing compounds, used to remove residual chlorine in water.
(3)Impregnated activated carbon for chemical adsorption
- Gas adsorption type: impregnated with acid and alkali compounds, used to adsorb specific industrial gases, such as ammonia (NH3) and sulfur dioxide (SO2).
- Organic adsorption type: impregnated with oxidants or reductants, used to adsorb and decompose organic compounds.
(4)Catalyst carrier
- Catalytic reaction type: impregnated with metal oxides or precious metals, used for industrial catalytic reactions, such as organic synthesis, waste gas treatment, etc.
4)Classification by impregnation method
(1)Wet impregnation
- Solution impregnation: immersing activated carbon in a solution containing the impregnant, commonly used for impregnation of metal salts or oxidants.
- Spray impregnation: The impregnant is evenly coated on the surface of the activated carbon by spraying, which is suitable for applications with lower impregnation amounts.
(2)Dry impregnation
- Gas phase impregnation: The activated carbon is exposed to the gas phase of the impregnant so that it is adsorbed on the surface of the activated carbon. It is often used for the application of volatile impregnants.
- Solid mixing method: The activated carbon is mixed with a solid impregnant, and the impregnant is covered on the surface of the activated carbon by heating or stirring.
Contact heycarbons to get price and learn more
Preparation and application of loaded impregnated activated carbon
The application scope of activated carbon as a carrier is much wider than that of activated carbon itself as a catalyst. The application scope of activated carbon as a carrier is not as limited as that of alumina.
It can load precious metals (such as Pt, Pd, Pu, Ph, Re, Os, Ir, etc.), sulfides (such as MnS, MoSe, wsHgS, ZnS, CuS, CdS), halides (AICl;, alkaline earth metals, chlorides, etc.), inorganic acids, etc. It is mainly used for hydrogenation or synthesis in pesticides, medicines, and spices, production of polyesters, polyurethanes, etc. in plastics and chemical fibers, and dehydrogenation of alicyclic compounds to aromatic compounds.
Among them, the most common application is the use of activated carbon to load rare precious metals, because one of the benefits of using activated carbon to load precious metals is the convenient recovery of precious metals, such as heating and burning the used catalyst.
The general process of using activated carbon as a carrier is to first impregnate the metal salt onto the activated carbon, and then heat the activated carbon loaded with precious metals. For example, the loading of Pt is to first load the platinum salt, and then perform pyrolysis and decomposition treatment.
The resulting P is loaded on the activated carbon in the form of tiny particles, but for other transition metal salts such as iron salts, the corresponding metal oxides are obtained after high-temperature heat treatment. In a catalytic environment with high acid-base strength such as the reduction reaction of nitrobenzene, alumina and silicon oxide molecular sieve carriers will not be able to withstand such an environment, but activated carbon carriers do not have such problems.
Moreover, in reactions such as methanol carbonyl synthesis of acid and ethanol carbonyl synthesis of propionic acid, activated carbon has better activity than SO2, A1Oa, molecular sieves and polymer carriers!
Conditions for activated carbon as a catalyst carrier
Activated carbon as a carrier has the following advantages: low price, high acid and alkali resistance, stable properties, developed pore structure, huge specific surface area, and excellent adsorption performance.
In addition, through the combustion of the carbon carrier, the precious metals loaded on the activated carbon are easier to recover, and the properties of the catalyst are also affected by the surface area, pore structure and surface functional groups of the activated carbon. These parameters of the carbon carrier can be modified by physical and chemical treatment methods, so that the catalyst has a larger adjustment and adaptability range.
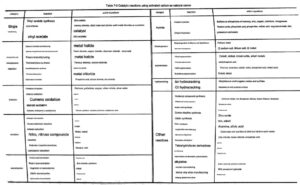
Therefore, the application of activated carbon as a carrier is becoming more and more extensive. The types of reactions catalyzed by catalysts with activated carbon as a carrier include: halogenation, redox, resin monomer manufacturing, polymerization, isomerization and other reactions. The table shows some catalytic reactions of activated carbon as a catalyst carrier.
Application technology of supported activated carbon catalysts.
1)Activated carbon supported acid catalysts and their applications
Using activated carbon supported acid as a catalyst has the advantages of high catalytic activity, good selectivity, convenient operation, less equipment corrosion and less environmental pollution.
Activated carbon supported acid catalysts can be widely used in reactions such as esterification. The preparation of activated carbon supported p-toluenesulfonic acid catalyst can be prepared by the following process: first, 10% dilute nitric acid is used to elute the activated carbon of 400-600 mesh, then wash it with water until it is neutral, soak it in distilled water, and then reflux it with deionized water for 2h, filter it under reduced pressure, and dry it at 150℃ for 3h.
The obtained clean activated carbon is refluxed and adsorbed with a certain concentration of p-toluenesulfonic acid (TsOH) solution for 12h, filtered under reduced pressure and dried, and finally activated at (120±2)℃ for 2h to obtain a series of catalysts TsOH/C with different solid loadings.
Ethyl acetate is the basic raw material for chemical and pharmaceutical production, and is also an important intermediate for dyes and fragrances. The traditional preparation method is the esterification of acetic acid and ethanol under the catalysis of concentrated sulfuric acid.
Although the esterification process is fast, the ester yield is low (70%~80%), and the post-reaction treatment process is complicated, there is “three wastes” pollution, and concentrated sulfuric acid not only corrodes equipment in industrial production, but also causes side reactions, such as dehydration and polymerization of alcohols.
In order to improve the ester yield and avoid corrosion to equipment, p-toluenesulfonic acid can be used instead of concentrated sulfuric acid to prepare ethyl acetate, but because p-toluenesulfonic acid is easily lost with ethyl acetate during the reaction, the catalytic cost is greatly increased, and the post-treatment of this process is still very complicated.
Studies have shown that compared with the non-solid-supported p-toluenesulfonic acid process, the use of cheap and readily available activated carbon-supported p-toluenesulfonic acid as a catalyst has the advantages of less catalyst dosage, long service life, high ester yield, simple post-reaction treatment process, no pollution to the environment, no corrosion to equipment, and the esterification reaction can be operated both intermittently and continuously, so it has gradually attracted widespread attention.
Using activated carbon as a carrier, an activated carbon-supported p-toluenesulfonic acid catalyst was prepared by an impregnation method. It was found that its catalytic effect on the synthesis of triacetin was better than that of the commonly used esterification catalyst, with a yield of more than 92%, 15% higher than that of sulfuric acid as a catalyst, and 10% higher than that of p-toluenesulfonic acid as a catalyst, and the product quality reached the standard of premium products.
In addition, activated carbon-supported acid catalysts can also be used in desulfurization. The effect of inorganic acid treatment on the performance of activated carbon carriers used for adsorption desulfurization of FCC gasoline was investigated.
The results showed that the desulfurizer treated with nitric acid (AC-NH) had better desulfurization performance than the desulfurizer treated with sulfuric acid and phosphoric acid (AC-SH, AC-PH).
When the nitric acid concentration was 65%, the activation temperature was 80℃, the roasting temperature was 250℃, the adsorption desulfurization temperature was 120℃, and the oil-agent ratio was 1.0, the desulfurization rate could reach up to 90.43%.
At the same time, the changes in the adsorption desulfurization performance of the activated carbon carrier after being treated with an oxidant were also studied. Compared with the desulfurizer treated with hydrogen peroxide (AC-H2O2), the desulfurizer treated with ammonium persulfate solution (AC-AP) had better adsorption performance.
When the concentration of ammonium persulfate solution is 10%, the activation temperature is 80℃, the calcination temperature is 250℃, the adsorption desulfurization temperature is 120℃, and the oil-agent ratio is 1.0, the desulfurization rate can reach up to 92.13%.
Moreover, GC-FPD analysis shows that the acid-treated desulfurizer and the oxidant-treated desulfurizer will preferentially adsorb and remove benzothiophene in gasoline; differential thermal thermogravimetric analysis experiments show that the activated carbon carrier and the nitric acid-treated desulfurizer are decomposed in two steps after heating, and the mass of the activated carbon carrier is basically constant between 100~600℃;
there is no obvious weight loss step of the activated carbon carrier after desulfurization; X-ray diffraction analysis shows that the surface acid content of the activated carbon carrier increases significantly and the alkali content decreases after nitric acid treatment; after calcination at 350℃, nitric acid treatment, and ammonium persulfate solution treatment, the specific surface area, pore volume, average pore size and pore distribution of the activated carbon carrier do not change much, indicating that the above activation treatment has little effect on the internal pore structure of the activated carbon carrier.
2)Activated carbon-supported metal catalysts and their applications The metals supported on activated carbon mainly include vanadium (V), manganese (Mn), copper (Cu), iron (Fe), cobalt (Co), nickel (Ni), platinum (Pt), titanium (Ti), etc. So far, many different types of metal catalysts supported on activated carbon have been industrially applied.
(1) Activated carbon-supported iron catalysts and their applications Iron is the most common metal in daily life and is cheap and easy to obtain, so it has been widely studied and has also been used in the field of flue gas desulfurization. Studies have shown.
When AC is loaded with iron before carbonization, the number of basic groups on the surface of AC increases significantly with the increase of iron content, while the acidic functional groups decrease slightly; while for materials loaded with iron after activation, the number of acidic and basic functional groups on the surface does not change significantly.
At low temperature, the adsorption capacity of SOz increases with the increase of iron content, and the good dispersion of iron is beneficial to improve the adsorption and oxidation of SO by the material. Studies have confirmed that the adsorption capacity of the material for SO2 will increase
when NO is present in the reaction gas. studied the desulfurization performance of Fe/AC under 423~523K conditions and showed that within the experimental temperature range, the removal capacity of the material for SO2 increases with the increase of temperature.
At 473K, the adsorption capacity of Fe/AC for SO2 is much higher than that of AC or Fe0₃ alone. The programmed temperature desorption experiment found that after the desulfurization process, HSO and Fez(SO:)3 were generated on the surface of the material.
The AC-based desulfurization catalyst with iron oxide loaded on the surface has strong regeneration ability. The iron oxide is not easily reduced during the regeneration process, and Fe/AC has a relatively long service life.
(2)Activated carbon-supported Cu catalyst and its application The research on CuO catalyst loaded on activated carbon is also extensive.
Compared the performance of CuO, FezO₃ and V₂〇; loaded on AC under the same experimental conditions for the simultaneous removal of SO2, NO and HCl from flue gas. The results show that the catalytic process of SO on metal oxides includes three stages: SO2 adsorption to form sulfite, sulfite oxidation to form sulfate, and sulfate decomposition to release SO2.
Moreover, it was found in the experiment that CuO/AC only acts as an adsorbent, and copper oxide deactivates the catalyst due to copper sulfate during the catalytic process.
In addition, studies have shown that AC has high catalytic activity at low temperatures as a catalyst carrier, and acid treatment is conducive to promoting the uniform dispersion of active substances loaded on AC and improving its chemical activity.
In this regard, Tseng et al. conducted relevant research on the catalytic oxidation of SO2 by CuO/AC. The conclusion of the study pointed out that the acid treatment process, the loaded metal and the size of the carrier particles are all factors that affect the catalytic activity of the CuO/AC catalyst.
The two processes of metal loading and acid treatment can promote the adsorption and transfer of SO2 and thus improve the oxidation activity of the material. Therefore, relatively speaking, the influence of the surface chemical properties of AC is greater than the influence of the physical properties of the carrier (such as pore structure).
A series of adsorption desulfurizers were prepared by isovolumetric impregnation method with activated carbon as carrier and CuO, ZnO and nitric acid as modifiers. The adsorption effect of this series of desulfurizers in diesel adsorption desulfurization was evaluated by static adsorption method and fixed bed dynamic adsorption method.
The results show that activated carbon-based adsorbents can effectively remove sulfur compounds from diesel. The adsorption desulfurization performance of modified activated carbon is better than that of original activated carbon (AC).
The desulfurization performance of activated carbon loaded with CuO (CuO/AC) is better than that loaded with ZnO (ZnO/AC). The desulfurization performance of activated carbon mixed with CuO/ZnO (CuO-ZnO/AC) is not much different from that of single loading of CuO or ZnO.
The desulfurization performance of activated carbon activated by nitric acid and then loaded with CuO (AC-N-A) is greatly improved, and its desulfurization performance is better than that directly loaded with CuO. Activated carbon loaded with CuO and ZnO is mainly used for the removal of dibenzothiophene and its derivatives in diesel.
The results of surface acid-base functional group determination show that the surface alkaline functional group content of the prepared desulfurizer is greater than the surface acidic functional group content, and the surface acid content is: AC-N-A>Cu0-ZnO/AC>Cu0/AC>ZnO/AC>AC, and the surface alkalinity is: CuO-ZnO/AC>ZnO/AC>CuO/AC>AC-N-A>AC.
The specific surface area, pore volume and average pore size of activated carbon are not greatly affected by calcination and nitric acid activation, and its most probable pore size is mainly concentrated between 2 and 3 nm.
Differential thermal-thermogravimetric analysis shows that when heated to 350℃, zinc nitrate crystals and copper nitrate crystals are almost completely decomposed into CuO and ZnO at 350℃, and activated carbon has good thermal stability between 200 and 550℃. X-ray diffraction analysis shows that the desulfurizer CuO/AC calcined at 350℃ has a diffraction peak of Cu2O at 350℃, which is because C reduces part of CuO to CuO;
The diffraction peak of elemental Cu appears at calcination at 500℃ and 700℃, which is because part of Cu oxide is reduced to elemental Cu by C; the diffraction peak of Cu20 in CuO/AC disappears after adsorption desulfurization, which may be due to the participation of CuzO in the removal of sulfur;
No diffraction peak of Zn in any phase is found in the desulfurizer ZnO/AC, which indicates that the active components are uniformly dispersed on the surface of activated carbon. The above desulfurizers were used for FCC diesel adsorption desulfurization evaluation results: the adsorption saturated sulfur capacity of desulfurizers CuO/AC, ZnO/AC, CuO-ZnO/AC and AC-N-A were 0.587%, 0.531%, 0.596% and 0.808% respectively;
The desulfurizers were used for model compound adsorption desulfurization evaluation results: AC, CuO/AC, ZnO/AC and AC-N-A had adsorption saturated sulfur capacity of thiophene of 0.261%, 0.325%, 0.298% and 0.470% respectively, and the adsorption saturated sulfur capacity of benzothiophene was 1.076%, 1.354%, 1.251% and 1.826% respectively.
The desulfurizers can selectively adsorb and remove benzothiophene. The adsorption saturated sulfur capacity of the prepared desulfurizers is: AC-N-A>CuO-ZnO/AC>CuO/AC>ZnO/AC>AC. The static adsorption desulfurization evaluation results show that when the CuO loading is 4.0%, the ZnO loading is 2.0%, the oil-agent ratio is 1.0, the immersion time is 2h, and the desulfurization temperature is 80℃, the maximum desulfurization rates of desulfurizers CuO/AC, ZnO/AC, CuO-ZnO/AC and AC-N-A are 45.27%, 44.40%, 45.76% and 60.95%,
Respectively. The evaluation results of fixed bed dynamic adsorption desulfurization show that when the CuO loading is 4.0%, the ZnO loading is 2.0%, the calcination temperature is 350℃, the calcination time is 2.0h, the desulfurization temperature is 80℃, the air velocity is 2.0h1, and the oil-agent ratio is 1.0,
the maximum desulfurization rates of desulfurizers CuO/AC, ZnO/AC and CuO-ZnO/AC are 46.98%, 43.91% and 47.82% respectively; when the CuO loading is 4.0%, the calcination temperature is 350℃, the calcination time is 2.0h, the desulfurization temperature is 100℃, the air velocity is 2.0h-1, and the oil-agent ratio is 1.0, the maximum desulfurization rate of desulfurizer AC-N-A is 70.60%. Through the regeneration study of desulfurizer AC-N-A, it is found that the use of gas purge thermal regeneration and organic solvent elution regeneration has a certain regeneration effect on the deactivated desulfurizer.
(3)Activated carbon-supported Ti catalyst and its application TiO has the photocatalytic performance of reducing energy consumption and saving energy, so its research has received more and more attention and has become a research hotspot in recent years.
At present, the research on TiOz is basically focused on photocatalysis, while there are not many studies on the thermodynamic reactions in which it participates. In terms of environmental protection, the research on TiOz is dominated by wastewater treatment.
Since the photocatalytic function of TiOz is not selective, it is suitable for the treatment of various wastewaters such as phenol-containing wastewater and dye wastewater. With the deepening of research in this area, TiO2 is expected to achieve industrialization in the field of wastewater treatment.
In terms of gaseous pollutant treatment, studies have found that TiO2 has shown broad application prospects in the treatment of indoor formaldehyde air pollution. This application deserves in-depth study, and related products are currently hot-selling in the market. In the field of flue gas desulfurization.
there have been reports at home and abroad on the use of composite materials of titanium dioxide and activated carbon as desulfurizers, and a wave of research on the preparation technology of TiO2 and AC composite materials has emerged.
(4)Activated carbon loaded with other metal catalysts and their applications Research on AC-based desulfurization catalysts of other transition metals (such as Lu and alkali metals or alkaline earth metals (such as potassium and calcium) is also in-depth.
In view of the fact that traditional flue gas desulfurization methods usually use alkali metal or alkaline earth metal oxides or hydrides as desulfurizers, some researchers load such desulfurizers on activated carbon to simultaneously play the advantages of desulfurizers and AC.
This is similar to the desulfurization of pyrolusite, and the loading of manganese oxides can also achieve flue gas desulfurization. Ducona Company and EPA in the United States studied 48 metal oxides and found that within the boiler flue temperature range, Fe, Co, Ni, Cr, Cu, Ce oxides can not only effectively remove SO2, but also the adsorbent itself can be well regenerated, and the adsorption rate is high, which has good practicality.