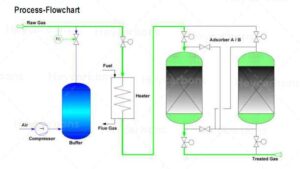
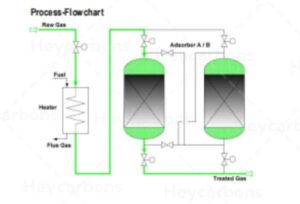
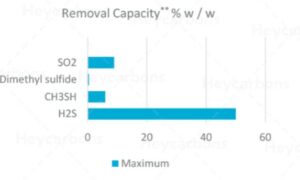
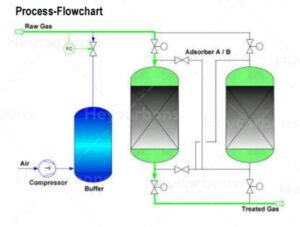
Hydrogen sulfide and mercaptans can be separated from the gas by different types of activated carbon.
The selection of the most suitable process and its associated activated carbon type is difficult and depends on the chemical composition of the gas as well as physical parameters such as humidity and temperature.
Generally, 5 different types of activated carbon for h2s removal can be chosen:
- Pelletized activated carbon.
- KI impregnated activated carbon.
- NaOH impregnated activated carbon.
- KOH impregnated activated carbon.
- Catalytic activated carbon.
- Iron oxide desulfurizer.
Tips are given below to choose the best type of activated carbon.
Gas temperature
• 10–70°C is preferred
• <10°C working layer extension
=> lower elemental sulfur loading
• >70°C leads to by-product formation
=> formation of SO2 and H2SO4
=> corrosion issues for downstream equipment
1)KI impregnated activated carbon for h2s removal
*The presence of 2 times the stoichiometric amount of oxygen is necessary to achieve the conversion of hydrogen sulfide to elemental sulfur.
*If the air flow interrupted irreversible damage to the carbon bed occurs. this damage cannot be reversed by the injection of more air.
*A prerequisite for high elemental sulfur loading is an adequate mixture.
*When the relative humidity is higher than 70%, heating of the gas is required.
*The conversion of H2S and mercaptans to elemental sulfur occurs through catalytic oxidation inside the pore structure.
*KI impregnated activated carbon Working Temperature of the gas :
• preferable temperatures of 10 – 70°C
•< 10°C extension of the working layer => lower loading of elemental sulfur
•> 70°C causes formation of by-products => SO2 and H2SO4 formation, => corrosion problems in the downstream equipment
* KI impregnated activated carbon for flue gas desulfurization is that the iodine during the reaction can catalytically oxidize sulfur dioxide to sulfuric acid, reduce iodine to hydrogen iodide, and hydrogen iodide is oxidized to iodine on the activated carbon, thereby cyclic reaction , greatly improving the adsorption capacity of activated carbon for sulfur dioxide.
Active centers are formed on the carbon surface, thereby promoting catalytic oxidation. By measuring the accumulation amount of sulfur trioxide on activated carbon at different times, it was found that the whole process can be divided into two stages with different reaction mechanisms.
When the accumulation amount of sulfur trioxide is small, the adsorption of sulfur dioxide and oxygen by sulfur trioxide is insufficient. After the accumulation of sulfur trioxide reaches a certain level, it becomes an inhibitor.
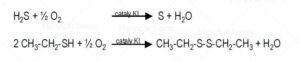
H2S + ½ O2 (catalyst KI)→ S + H2O
2CH3-CH2-SH + ½ O2 (catalyst KI)→ CH3-CH2-S-S-CH2-CH3 + H2O
2) NaOH impregnated activated carbon sulfur removal
*NaOH impregnated activated carbon does not require oxygen in the gas when used.
*H2S removal is a chemical adsorption process
*described by the following equation:
H2S+2NaOH → Na2S + 2H2O
It is easy to see that NaOH is used to neutralize the acidic H2S. The adsorption capacity is limited by the amount of NaOH,
which also reacts with all other acidic components present in the gas to be treated.
*The mercaptans are converted to sodium salts according to the following equation:
R-SH + NaOH → R-SNa + H2O
*A For KI impregnation of activated carbon, the gas humidity should not exceed 70% rh.
*In addition to the NaOH impregnation type, there are other alkaline types that can be used as impregnants.
3)KOH impregnated activated carbon for h2s removal
2KOH+H2S → K2S+2H2O
When using KOH-impregnated desulfurization activated carbon, ambient humidity, temperature, and oxygen content have a significant impact on its performance. The following are specific requirements:
1. Humidity requirements
Relative humidity (RH): The relative humidity of KOH-impregnated desulfurization activated carbon in the use environment should be maintained between 40-60%. Excessive humidity may cause KOH to react with moisture to form potassium hydroxide solution, reducing the desulfurization effect of activated carbon. Too low humidity may affect the efficiency of the desulfurization reaction.
2. Temperature requirements
Working temperature: During the use of KOH impregnated desulfurization activated carbon, the optimal working temperature range is usually 20-40°C. Too high a temperature may cause the desulfurizer to fail, and too low a temperature may reduce the reaction rate.
Stable temperature: It is also important to maintain the stability of the ambient temperature. Excessive temperature fluctuations will affect the continuity and efficiency of the desulfurization reaction.
3. Oxygen content requirements
Oxygen content: During use, the oxygen content should be controlled at a low level, usually less than 5%. A high oxygen environment may cause activated carbon to oxidize and reduce its desulfurization ability.
Gas flow rate: In practical applications, the oxygen content and contact time are usually controlled by adjusting the gas flow rate to optimize the desulfurization effect.
Summarize:
Humidity: relative humidity 40-60%
Temperature: Optimum working temperature 20-40℃, maintain temperature stability
Oxygen content: less than 5%
The control of these environmental conditions can effectively improve the performance of KOH-impregnated desulfurization activated carbon and ensure its desulfurization effect and service life in actual use.
4)Catalytic activated carbon sulfur removal H2S
The use of catalytic activated carbon requires the presence of oxygen in the gas. The humidity of the gas may be higher (maximum 90% relative humidity) and the H2S content is limited to a maximum of 50ppm, without damaging the activated carbon.
The activated carbon also performs well with higher H2S content, but the oxidation of the H2S may be incomplete.
H2S + 2O2 → H2SO4
*In these cases, elemental sulfur may be present and cannot be removed from the carbon by water washing.
*Within the above limits, high temperatures and high moisture content give the best performance.
*The washed carbon can be reused for the next adsorption cycle.
*The capacity of the activated carbon decreases with the number of cycles.
*This activated carbon can also remove ammonia when sulfuric acid is formed on the carbon surface.
*Plants that meet these characteristics and high relative humidity can recommend the use of Heycarbons catalytic activated carbon for use in odor control devices where ammonia is always generated.
5)Iron oxide desulfurizer for h2s removal
In addition to activated carbon, iron oxide desulfurizer can also be used for H2S removal.
Working principle:
Fe2O3·XH2O+H2S→Fe2S3·XH2O+H2O(desulfurization)
Fe2S3·XH2O+O2→Fe2O3·XH2O+S (regeneration)
Working environment :
Therefore, the suitable gas humidity range for IRON OXIDE DESULFURIZER is a relative humidity of 20~100%, and the best is close to saturated water vapor, but it is strictly forbidden to bring water and free water to the bed, as long as the gas contains an appropriate amount of water vapor. Too dry or too wet will affect desulfurization efficiency.
- Air flow speed: 300~800 h-1
- Temperature: 15~80℃
- Pressure: normal pressures~8.0Mpa
- Contact time: ≥45 second
- O2 content 0.2% and 0.4% with different working H2S capacity.
It is suitable for rough desulfurization when H2S is greater than 50PPM. It is more cost-effective than activated carbon and can be combined with activated carbon for back-end fine desulfurization.
Scenes to be used:
This desulfurizer is suitable for natural gas, water gas, semi-water gas, air coal, Removal of H2S from gas, coke oven gas, shift gas, steel plant raw gas, biogas, petrochemical industry and other gases.
Related Products: