Activated Carbon - Ultimate Buying Guide
Outline:
What is activated carbon?
Classification of activated carbon
Production process of activated carbon
Application of activated carbon
Regeneration and treatment of activated carbon
How to choose the corresponding activated carbon according to the application?

Contents
Chapter 1
Introduction to Heycarbons activated carbon
What is Activated Carbon?
Activated carbon/Activated charcoal CAS No.:
CAS No.:64365-11-3
CAS No.:7440 44 0

What is Activated Carbon?
Activated carbon is a carbon material with a developed pore structure, large specific surface area, and strong selective adsorption capacity. Under certain conditions, it can adsorb, remove, purify, refine or recover one or some substances in liquid or gas to achieve product refinement and environmental purification.
Raphealvon Ostrejko applied for British patents B.P.14224 and B.P.18040 in 1900, and first studied and developed the production of activated carbon with adsorption capacity by CO or water vapor activation reaction, and successfully applied it to gas masks. In 1911, Austrian Fanto Company and Dutch Norit Company first produced powdered activated carbon for decolorization of sugar solution.
Today, activated carbon has been widely used in military industry, chemical industry, food, light industry, medicine, pharmaceutical environmental protection and water treatment and other industries and all aspects of life. With the development of science and technology and the improvement of people’s living standards, activated carbon has become an indispensable carbonaceous adsorption material in modern industry, ecological environment and people’s lives.

Activated carbon/Activated charcoal CAS No.:
CAS No.:64365-11-3
| Product application: | Activated carbon (64365-11-3) uses: 1. Deodorant; decolorant; deodorant; purifier in food production. 2.Widely used in decolorization and purification of beverages such as glucose, sucrose, maltose, oil, fruit juice and wine, removal of colloidal substances and water treatment. GB 2760-96 lists it as a processing aid. The dosage depends on production needs. The general maintenance time is 30 minutes. |
| Production method and others: | Production method and others: Activated carbon (64365-11-3) preparation method: 1.Organic raw materials that can be carbonized and activated (including sawdust, lignite, charcoal fiber residue, peat, fruit shells, animal bones, petroleum coke, etc.) are carbonized and activated at high temperature in an activated gas such as water vapor or carbon dioxide with or without inorganic salts. 2. It can be made by carbonizing a chemical activator such as phosphoric acid or zinc chloride at high temperature and then washing with water to remove the chemical activator. |
CAS No.:7440 44 0
| Product application: | 1. Activated carbon is mainly used in the following aspects. 1. Gas purification For example, activated carbon is used to recover solvents from air containing solvent vapors; activated carbon filtration is used to deodorize air; or it is used in gas masks and industrial respirators to defend against toxic substances, etc. 2. Gas separation For example, benzene is recovered from city gas, gasoline, propane and butane are recovered from natural gas, and it is used to treat waste gas in Fischer-Tropsch synthesis to recover hydrocarbons, etc. 3. Liquid phase adsorption For example, activated carbon adsorption is used to decolorize sugar solution in the sugar industry, activated carbon is used to decolorize organic substances in the chemical industry, and activated carbon is used to purify organic impurities in electroplating baths to ensure the quality of electroplating surfaces or for wastewater dephenolization, etc. 4. Catalyst or catalyst carrier For example, it is used as a catalyst for desulfurization of industrial gas and production of phosgene, etc. Therefore, activated carbon is widely used in various aspects such as sugar making, wine processing, oil refining, pharmaceuticals, reagents, metallurgy, water purification, environmental protection, recovery of solvents and other vapors. |
| Production method and others: | Activated carbon is made from sawdust, branches, charcoal, fruit shells, coal and other raw materials through carbonization and activation. The carbon-containing material is first heated in an airtight condition to remove volatiles (water and some tar) to form macroporous carbon. There are two methods for further activation: 1. Steam; gas activation. The more commonly used activators are carbon dioxide and water vapor. 2. Chemical activation. The activators are carbonates, sulfates, nitrates, etc., which can release gases (such as carbon dioxide and oxygen) at high temperatures. Acid oxidants such as nitric acid, sulfuric acid, and phosphoric acid can also be used. The method of treating carbon with a concentrated zinc chloride solution has also been widely used. Zinc chloride method, raw material consumption (kg/t) Sawdust 3900 Zinc chloride 500 Hydrochloric acid (31) 750 |
Activated carbon is mainly a porous adsorbent made from high-carbon materials such as charcoal, sawdust, various fruit shells (coconut shells, apricot shells, walnut shells, etc.), coal and petroleum coke, etc., through carbonization and activation.
Activated carbon is basically a non-crystalline substance, which is composed of fine graphite crystals and hydrocarbon parts that connect them together. The gaps between its solid parts form pores, which give activated carbon its unique adsorption properties.
You will get a quote in 24 hours
Chapter 2
Heycarbons Activated Carbon Products Classification
Activated Carbon Physical Structure
Pore of Activated Carbon Structure
Specific surface area
Classification of activated carbon production

Heycarbons Activated Carbon Physical Structure
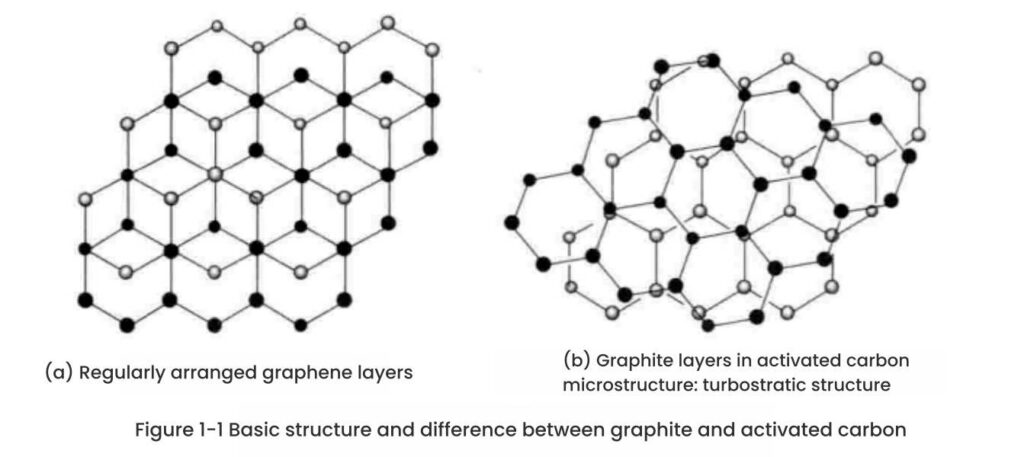
Basic crystal structure of activated carbon Activated carbon is an adsorption material with carbon as the main component. It has a complex structure. It does not have a molecular structure with carbon atoms arranged in a certain pattern like graphite and diamond, nor does it have a complex macromolecular structure like general carbonized materials.
It is generally believed that activated carbon is a block and pore structure composed of carbon microcrystals similar to graphite and amorphous carbon. X-ray diffraction analysis shows that the activated carbon structure contains graphite microcrystals, which are arranged irregularly and have a size of 1~3nm. According to the X-ray analysis data of Riley, in addition to graphite microcrystals, activated carbon also contains amorphous carbon.
The original raw materials of activated carbon, such as wood and coal, have formed microcrystalline carbon (the basic crystal of activated carbon) between some carbon atoms in the activated carbon after carbonization and activation. However, its surface network structure does not adopt a regular layered structure like graphite, but forms a chaotic layer structure as shown in Figure 1-1(b).
In addition to microcrystalline carbon, activated carbon precursors still have some uncrystallized carbon after carbonization and activation. Activated carbon is considered to be a multiphase material composed of microcrystalline groups and other single mesh planes that do not form parallel layers and irregular carbon.
Pore of Activated Carbon Structure
1.The morphology of pore structure. The pores of activated carbon are the pores produced after various carbon-containing compounds and disordered carbon are removed between the basic microcrystals (sometimes part of the carbon is removed from the graphite layer of the basic microcrystals) during the activation process.
The size, shape and distribution of the pores vary depending on the raw materials, carbonization and activation processes and methods used to prepare the activated carbon. Different pore structures can play corresponding functions. In 1960, Dubinin divided the pores of activated carbon into three categories: macropores (pore diameter greater than 50nm), mesopores (or transitional pores, pore diameter 2~50nm) and micropores (pore diameter less than 2nm).
This scheme has been accepted by the International Union of Pure and Applied Chemistry (IUPAC). In activated carbon, these three types of pores of different sizes are interconnected and present a tree-like structure, as shown in Figure 1-3.
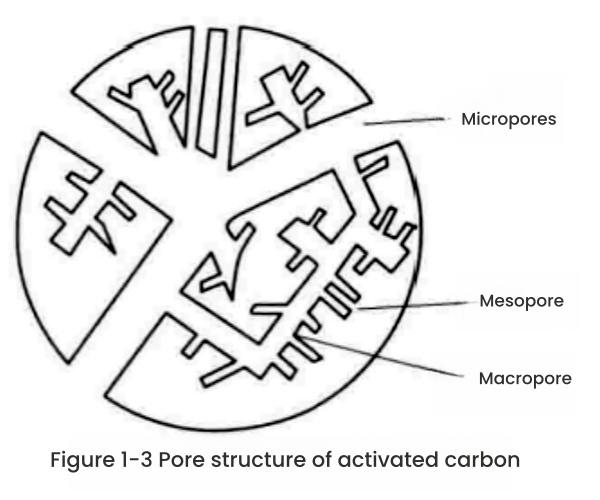
High-resolution transmission electron microscopy studies have shown that the micropores in activated carbon are molecular-sized gaps between the bent and deformed aromatic ring layers or bands in the activated carbon microcrystalline structure. The shapes of the pores vary. Using different research methods, it was found that some are capillary pores with one end closed or two ends open, some pores have a narrow entrance (bottle-shaped pores), and some are more or less regular slit-shaped pores, V-shaped pores, etc. between two planes.
The inner surface of the macropores in the Dubinin classification can undergo multilayer adsorption, but in activated carbon, due to its small proportion, most of it serves as a passage for the adsorbate molecules to enter the adsorption site, but it can determine the adsorption rate, so it is also very important in practical applications.
In many cases, transition pores are the same as macropores, and they also serve as passages for adsorbates to control the adsorption rate, but the role of transition pores is not simple, it can also serve as an adsorption site for macromolecules that cannot enter the micropores.
Most of the adsorption of activated carbon is carried out through micropores, so micropores also determine the adsorption amount of activated carbon. The generation of micropores, corresponding to a trace amount of mass loss, can form a very large specific surface area.
2.Calculation of pore volume. In activated carbon, the activation process increases its pore volume. It can be considered that the development of pores determines the increase in pore volume.
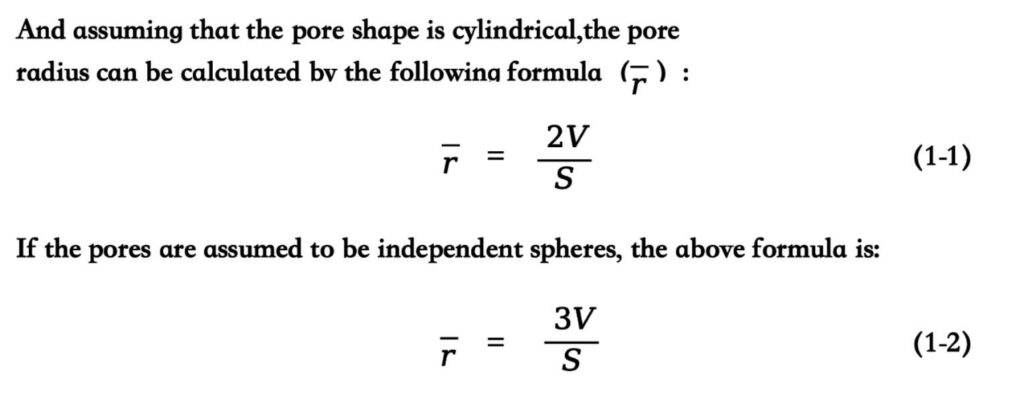
If the shape of the pores is a crack-like shape composed of parallel planes, the pore radius is equivalent to the plane spacing.
If the specific surface area (S) and pore volume (V) are determined and the pore shape is assumed to be cylindrical, it can be used The following formula calculates the pore radius (r):
3.Determination of pore size distribution. If you want to understand the pore structure in detail, pore size distribution is the best means. Half of the performance of activated carbon can be expressed by pore size distribution. Usually, the methods for determining pore size distribution include mercury intrusion, electron microscopy, capillary condensation, molecular sieve, X-ray small angle scattering, etc. Mercury intrusion is a more commonly used method. Its principle is to use the fact that mercury does not wet the pore wall of activated carbon, so it can be pressed into the pores, and the following formula is established:
r p = – 2 rcos θ
In the formula, r is the radius of the cylindrical pore; p is the pressure applied to mercury; In the formula, r is the radius of the cylindrical pore; p is the pressure applied to mercury; θ is the contact angle of mercury; γ is the surface tension of mercury.
Under pressure p, mercury should enter all pores with a radius greater than r, so the amount of mercury entering due to the increase in pressure can be measured, thereby measuring the size of each pore and then determining the pore size distribution. is the contact angle of mercury; γ is the surface tension of mercury.
Under pressure p, mercury should enter all pores with a radius greater than r, so the amount of mercury entering due to the increase in pressure can be measured, thereby measuring the size of each pore and then determining the pore size distribution.
Specific surface area
Adsorption occurs on the surface of solids, and the strength of an object’s adsorption capacity depends largely on the size of its specific surface area. There are many analytical methods that can be used to determine specific surface area, among which the BET method is the most commonly used.
In addition, there are also flow-through method, liquid phase adsorption method, and wetting heat method. In addition, the specific surface area can also be determined by small-angle X-ray scattering, but the BET method is still the most commonly used method for determining the specific surface area of activated carbon. The specific surface area of activated carbon determined by this method is generally 1000m²/g.
You will get a quote in 24 hours
Classification of Heycarbons activated carbon production
Activated carbon can be divided into different types according to the manufacturing method, appearance, function and pore size.
From the perspective of morphology, it can be divided into granular activated carbon and powdered activated carbon, and granular activated carbon can be divided into two categories: amorphous and morphological;
Based on the different raw materials, activated carbon types can be divided into charred wood, petroleum, coal and resin activated carbon;
Based on the different functions, types of activated carbon can be divided into liquid adsorption, catalytic performance, gas adsorption activated carbon;
Based on the manufacturing method, activated carbon types can be divided into physical method, chemical method and physical and chemical method activated carbon.
Specific classification and main uses are shown in Table 1-1 to Table 1-5.
Based on the appearance and shape, the current common activated carbon classification is shown in Table 1-1.
Table 1-1 Classification of commercially available activated carbon in different appearances and shapes
| Shape | Feature |
|---|---|
| Powdered activated carbon | Activated carbon with a particle size of less than 0.18nm (about 80 mesh) accounts for the majority. In addition to powdered activated carbon produced from sawdust, it also includes pulverized products of granular activated carbon, etc. learn more:https://heycarbons.com/powdered-activated-carbon/ |
| Pelletized Activated Carbon | Activated carbon is made by mixing raw materials and water into a column and then carbonizing and activating it. learn more:https://heycarbons.com/pelletized-activated-carbon/ |
| Granular activated carbon carbon | Coconut shell activated carbon and coal-based activated carbon belong to this category. The outer surface of activated carbon is angular due to crushing. learn more:https://heycarbons.com/granular-activated-carbon/ |
| Spherical carbon | Activated carbon produced by making carbonized materials into spheres and then activating them, and using spherical resin as raw materials learn more:https://www.youtube.com/shorts/UqetPcoVGTg |
| Hollow micro-spherical carbon | Most of them are made of resin, sometimes with a diameter of less than 50μm, and less powder is generated during use. |
| Fibrous activated carbon | Refers to activated carbon with a fiber diameter of 8~10μm made from fibrous raw materials. There are several types of activated carbon, such as filaments, cloth, and felt. |
| Honeycomb activated carbon | Extruded activated carbon in honeycomb shape |
| Activated carbon molding | There are products in which activated carbon powder is attached to a substrate such as paper, nonwoven fabric or sponge, and products in which activated carbon is processed alone or combined with other materials into various shapes. |
According to different raw materials, the classification is shown in Table 1-2.
Table 1-2 Types of commercially available activated carbon from different raw materials
| Type | Raw material |
|---|---|
| Wood activated carbon | Activated carbon made from sawdust, charcoal, etc. learn more:https://heycarbons.com/wood-activated-carbon/ |
| Coconutshell activated carbon | Activated carbon made from coconut shells, walnut shells, apricot kernels, etc. learn more:https://heycarbons.com/coconut-shell-activated-carbon/ |
| Coal-based activated carbon | Activated carbon made from lignite, peat, bituminous coal, anthracite, etc. learn more:https://heycarbons.com/coal-activated-carbon/ |
| Petroleum activated carbon | Asphalt-based spherical activated carbon made from asphalt and other raw materials |
| Regenerated carbon | Regenerated carbon is made from used waste carbon and is reactivated. It is different from raw carbon. |
According to different manufacturing methods, the classification is shown in Table 1-3.
Table 1-3 Types of activated carbon by different manufacturing methods
| Activation method | Activator |
|---|---|
| Chemical activated carbon | Chemicals such as zinc chloride, phosphoric acid, potassium hydroxide, sodium hydroxide, etc. |
| Strong alkali activated carbon | Potassium hydroxide, sodium hydroxide, etc. |
| Gas activated carbon | Water vapor, carbon dioxide, air, etc. |
| Steam activated carbon | water vapor |
Depending on the place where activated carbon is used, the classification is shown in Table 1-4.
Table 1-4 Classification of activated carbon in different places of use
| Scenes | Application |
|---|---|
| Gas phase | Exhaust treatment, air purification, solvent recovery, deodorization, gas separation, desulfurization and denitrification, process gas purification (carbon dioxide, compressed air, etc.), semiconductor gas purification, molecular sieve, radioactive gas retention, humidity control, fragrance adjustment, gas chromatography filler, gas analysis trap, freshness preservation, ozone removal, cigarette filter, natural gas adsorption storage, etc. learn more:https://heycarbons.com/activated-carbon-for-air-purification-application/ |
| Liquid Phase | Water treatment, highly purified water treatment, ultrapure water treatment, water purifiers, sewage treatment, factory wastewater treatment, decolorization and purification, odor removal, blood purification, free chlorine removal, gold recovery, brewing, detoxification, etc. learn more:https://heycarbons.com/activated-carbon-for-water-treatment-application/ |
| Catalyst | Catalysts, catalyst carriers, disposable pocket warmers, etc. learn more:https://heycarbons.com/impregnated-activated-carbon/ |
Activated carbon can also be classified based on its function, as shown in Table 1-5.
Table 1-5 Classification of activated carbon with different functions
| Activated carbon | Function |
|---|---|
| High surface area activated carbon | High specific surface area activated carbon with a specific surface area of more than 2500m²/g, produced by strong alkali activation method |
| Molecular sieve activated carbon | Very small pore size for separation of gases |
| Add activated carbon | Add chemicals such as metal salts on the surface of activated carbon for use in deodorization, catalysts, etc. |
| Biological activated carbon | One of the methods of water treatment. It forms a microbial film on the surface of activated carbon and purifies water through the decomposition of microorganisms. It is used in combination with ozone treatment for high-level water treatment. |
Chapter 3
Heycarbons Activated Carbon Functions
Activated carbon Chemical properties
Elemental composition
Function

Heycarbons Activated Carbon Chemical properties
Activated Carbon Elemental composition
1.Industrial analysis. For the determination of the amount of volatile matter contained in activated carbon and its raw material carbonized product, the method usually adopted is to place the sample in a platinum crucible, avoid contact with air, heat it at 900℃ for 7 minutes, calculate the percentage of heating loss to the original sample, and subtract the moisture value (drying loss) obtained by the simultaneous determination from this percentage to obtain the volatile matter content of the sample.
The ash content (strong heat residual) is determined by placing the dried sample in a porcelain crucible and placing it in a high-temperature electric furnace, adjusting its temperature to 800-900℃ to ash the sample, and the mass fraction of the residual substance is taken as the ash content. Fixed carbon is determined by taking the dry sample as 100%, and subtracting the ash content and volatile matter from the value obtained.

Since ordinary activated carbon is made at a temperature above 900°C, it has very little volatile matter. On the other hand, the carbonization temperature has a great influence on the volatilization of the raw carbonized material.
Experiments show that the content of volatile matter decreases with the increase of temperature; the carbonization reaction proceeds violently below 500°C and basically ends at 600-700°C. The fixed carbon content basically does not increase above 700°C, where the carbonization reaction ends, and this change basically corresponds to the volatile matter.
Ash content increases as the carbonization yield decreases. Ash content is an important indicator for selecting activated carbon raw materials. The inorganic components in the raw materials are almost not reduced during the carbonization process and finally remain in the charcoal.
Even if the ash content in the raw materials is only 1%, the ash content of the activated carbon will reach 10%. Since ash has no adsorption capacity, the adsorption capacity of this unit mass of activated carbon is about 10% lower than that of activated carbon with zero ash content. Therefore, in the process of selecting activated carbon, try to choose the one with the lowest ash content.
2.Elemental analysis. The elemental composition of activated carbon can be determined by an elemental analysis device (Table 1-6). The carbon content in activated carbon reaches more than 90%, which largely determines that activated carbon is a hydrophobic adsorbent. The oxygen content is generally a few percent, and there are two ways of existence.
One part exists in the ash, and the other part exists on the surface of carbon in the form of surface functional groups such as carboxyl groups. Oxygen-containing functional groups make activated carbon have a certain hydrophilicity, but it is not completely hydrophobic. The hydrophilicity of activated carbon enables it to replace the air in its own pores with water, and then adsorb organic matter dissolved in water, making it possible to use activated carbon for water treatment.
Table 1-6 Elemental composition of activated carbon
| Activated carbon | Carbon/% | Hydrogen/% | Sulfur/% | Oxygen/% | Ash/% |
|---|---|---|---|---|---|
| Steam activated carbon A | 93.31 | 0.93 | 0 | 3.25 | 2.51 |
| Steam activated carbon B | 91.12 | 0.68 | 0.02 | 4.48 | 3.7 |
| Zinc oxide activated carbon C | 90.88 | 1.55 | 0 | 6.27 | 1.3 |
| Zinc chloride activated carbon D | 93.88 | 1.71 | 0 | 4.37 | 0.05 |
| Zinc chloride activated carbon E | 92.2 | 1.66 | 1.21 | 5.61 | 0.04 |
① Experimental, trial-produced sulfur-added carbon.
Note: Nitrogen content is at trace levels.
The nitrogen and sulfur content in plant raw materials is usually very low. Most of the proteins and sulfides contained in the raw materials will be pyrolyzed and converted into gases and volatilized during the carbonization and activation process, but sometimes there will be trace amounts of residues. The remaining trace nitrogen atoms will improve the catalytic performance of activated carbon under certain conditions.
Due to the differences in raw materials and preparation processes, the ash content in activated carbon also varies significantly. Generally, wood-based activated carbon has a low ash content (generally <5%). When the raw material ash content is high, the raw material needs to be deashed before preparing activated carbon.
3.Harmful substances. Trace impurities in activated carbon must be minimized. Arsenic is the most important impurity in activated carbon. Arsenic exists in nature. If the arsenic concentration in the soil is too high, the arsenic concentration in the raw materials will also increase.Therefore, the arsenic concentration in the activated carbon produced using these raw materials will exceed the standard.
The above factors must be taken into account when selecting coconut shells and wood raw materials. The arsenic content must be strictly controlled when producing activated carbon.For the recent use of various waste gases as raw materials to produce activated carbon, it is also necessary to first detect whether it is contaminated by heavy metals.
Heycarbons Activated Carbon Function
The chemical functional groups, surface heteroatoms and compounds on the surface determine the surface chemical properties of activated carbon, and the surface chemical properties determine the adsorption characteristics of activated carbon. Different surface functional groups, heteroatoms and compounds have obvious differences in adsorption for different adsorbents, so studying the surface functional groups of activated carbon is of great help to its use function.
Activated carbon is a non-polar adsorbent. Due to its hydrophobicity, activated carbon can only adsorb various non-polar organic substances in aqueous solution and does not have the function of adsorbing polar solutes. However, through the introduction and modification of surface functional groups, it can have richer adsorption characteristics. Generally speaking, acidic compounds in the oxygen-containing functional groups on the surface of activated carbon are easy to absorb polar compounds, while alkaline compounds are easy to adsorb substances with weak polarity or non-polar substances.
The adsorption performance of activated carbon for adsorbents is changed by modifying the surface functional groups. Increasing the polarity of its surface functional groups can increase its adsorption capacity for polar substances. Correspondingly, increasing the non-polarity of the surface of activated carbon also increases its adsorption capacity for non-polar substances. Therefore, by changing the surface chemical properties of activated carbon, it can have a stronger and more comprehensive adsorption function.
You will get a quote in 24 hours
Chapter 4
Heycarbons Activated Carbon Types and Uses
Heycarb Activated Carbon
Main Types and Uses
Activated carbon application as gas phase adsorbent
Activated carbon application as liquid adsorbent
Activated carbon application as catalyst and catalyst carrier
Activated carbon other applications of activated carbon

Heycarbons Activated Carbon Main Types and Uses
As an adsorbent, the adsorption and catalytic properties of activated carbon have been extended to a wider range of fields and its uses are also broader.
Using activated carbon to absorb gas is an important method for air purification, odor removal, product recovery, etc. As people’s environmental awareness increases, the demand for activated carbon in the treatment of air pollution will increase. Usually, granular activated carbon is used for gas adsorption, and its strong adsorption capacity mainly comes from its developed microporous structure.
Activated carbon not only adsorbs a wide variety of gases at a fast speed, but most of the discarded activated carbon can be regenerated, and it itself has little pollution. Therefore, activated carbon has developed rapidly in indoor air purification.

Learn more about activated Carbon For Air Purification,VOCs,Natural gas desulfurization, biogas desulfurization,Air filter element,
Gas mask,Solvent recovery,Factory waste gas treatment: waste power plant, steel plant flue gas…
Activated Carbon For Air Purification Application
Heycarbons Activated carbon application as liquid adsorbent
As a liquid phase adsorbent, activated carbon was initially used in the industry as a decolorizing agent for refined sugar. Currently, in liquid phase adsorption, activated carbon is mainly used for decolorization and flavor adjustment in the food industry, water quality improvement in water treatment (treatment of various sewage, purification of tap water, water purification filter, etc.), decolorization and purification of pharmaceuticals in the pharmaceutical industry (such as penicillin production, etc.)
And it is also widely used in petrochemicals and rubber production. Almost all biological and chemical synthesis production processes will choose activated carbon as a refined adsorption material. Therefore, the development and promotion of activated carbon in separation, refining, purification, etc. should be a major topic of current research.

Learn More About Activated Carbon For Water Treatment,Wastewater Treatment,Water Filter…
https://heycarbons.com/activated-carbon-for-water-treatment-application/
learn more:Gold refining, Food decolorization, sugar decolorization, oil decolorization, glucose decolorization
Heycarbons Activated carbon application as catalyst and catalyst carrier
As a liquid phase adsorbent, activated carbon was initially used in the industry as a decolorizing agent for refined sugar. Currently, in liquid phase adsorption, activated carbon is mainly used for decolorization and flavor adjustment in the food industry, water quality improvement in water treatment (treatment of various sewage, purification of tap water, water purification filter, etc.), decolorization and purification of pharmaceuticals in the pharmaceutical industry (such as penicillin production, etc.)
And it is also widely used in petrochemicals and rubber production. Almost all biological and chemical synthesis production processes will choose activated carbon as a refined adsorption material. Therefore, the development and promotion of activated carbon in separation, refining, purification, etc. should be a major topic of current research.
learn more: Application as catalyst and catalyst carrier
Heycarbons Other applications of activated carbon
As the research gradually deepens, some special applications of activated carbon appear in other fields, and its development and utilization have brought many positive effects to our lives.
① Medicinal and medical use: As a high adsorption material, activated carbon can adsorb drugs, and release drug components slowly after oral administration to reduce the frequency of medication; activated carbon can also be used as an antidote and lipid-lowering drug. For example, it is used to treat gastrointestinal disorders, abdominal sepsis, blood filtration, blood dialysis, etc. to adsorb substances that are harmful and toxic to the human body.
② Metal selection: For example, it can be used to separate uranium from seawater by co-precipitation with a mixture of aluminum hydroxide and iron hydroxide.
③ Flue gas filtration and purification: filter tips for cigarettes and pipes.
④ Analytical technology: such as high vacuum technology used to adsorb trace residual gases.
⑤ Temperature control: used to make adsorption thermostats and obtain ultra-low temperatures.
⑥ Agricultural and forestry planting: used to slowly release agricultural fertilizers and pesticides in the soil, improve soil, regulate soil performance, and improve soil quality and ground temperature.
⑦ Energy field: used as hydrogen storage material, as an electrode material for batteries and supercapacitors.
Chapter 5
Heycarb Activated Carbon
Adsorption principle
Heycarbons Activated Carbon Adsorption principle
Activated carbon adsorption
Pore Distribution of Activated Carbon Structure
Activated carbon physical form
Activated carbon surface chemical functional groups
Activated carbon adsorbate
Activated carbon application conditions

Heycarbons Activated carbon adsorption
According to the different interaction modes between adsorbent and adsorbate, adsorption can be divided into physical adsorption and chemical adsorption. In terms of mechanism, physical adsorption is adsorption caused by van der Waals force, while chemical adsorption is adsorption that generates chemical bonds or is accompanied by charge transfer interaction.
In physical adsorption, the electron orbits do not overlap in the surface layer of the adsorbate and the adsorption medium; on the contrary, the overlap of electron orbits plays a vital role in chemical adsorption. In other words, physical adsorption basically occurs through the weak interaction between the adsorbate and the atoms on the surface of the adsorption medium; while chemical adsorption originates from the specific interaction between the electron orbits on the surface of the adsorption medium and the molecular orbits of the adsorbate.
Therefore, multi-molecular layer adsorption often occurs in physical adsorption; while chemical adsorption is a single-molecular layer. Moreover, chemical adsorption is accompanied by changes in the molecular binding state, and adsorption causes significant changes in the electronic state and vibration.
Through Fourier transform infrared spectroscopy, it can be observed that the adsorbate has undergone significant changes before and after adsorption. However, physical adsorption does not have such changes. The difference between physical adsorption and chemical adsorption is shown in Table 1-7.
Table 1-7 Differences between physical adsorption and chemical adsorption
| project | Physical adsorption | Chemical adsorption |
|---|---|---|
| Adsorbate | No selectivity | Be selective |
| Generates specific chemical bonds | none | have |
| Physical and chemical properties of solid surfaces | Can be ignored | Significant |
| temperature | Large adsorption capacity at low temperature | At a relatively high temperature |
| Adsorption heat | Small, equivalent to the condensation heat | Large, equivalent to the heat of reaction |
| Adsorption capacity | Monolayer adsorption capacity or more | Monolayer adsorption below |
| Adsorption rate | quick | slow |
| Reversibility | Reversible | There are irreversible situations |
Regarding the evaluation of adsorption phenomena, chemical adsorption is caused by characteristic effects and is difficult to be generally evaluated. It is necessary to conduct an evaluation corresponding to each adsorption system.
However, physical adsorption is not caused by the specific effect between the adsorbate and the surface system of the adsorption medium, so a general evaluation can be conducted.
Heycarbons Pore Distribution of Activated Carbon Structure
Granular activated carbon has a three-dispersed pore structure, that is, their pore sizes are very uneven, mainly concentrated in three size ranges: macropores, mesopores and micropores.
Macropores, also known as coarse pores, refer to pores with a radius of 100 to 200 nm. In macropores, steam will not undergo capillary condensation. There is no essential difference between the inner surface of the macropore and the non-porous carbon surface, and its proportion is very small, so its effect on the adsorption amount can be ignored. Macropores act as adsorption channels in the adsorption process.
Mesopores, also known as mesopores, refer to pores in which steam can undergo capillary condensation and cause hysteresis loops in the adsorption isotherm, and their radius is often between 2 and 100 nm. The size of mesopores is much smaller than that of macropores. Although there is no essential difference between its inner surface and the non-porous carbon surface, its specific surface area already accounts for a certain proportion, so it has a certain effect on the adsorption amount. But in general, it mainly acts as a coarse and fine adsorption channel.
Micropores have an effective radius of the same order of magnitude as the molecules of the adsorbed substance (less than 2nm), and are the most important pore structure of activated carbon, which determines the amount of adsorption. The inner surface of the micropores relatively avoids the overlap of the adsorption force field, resulting in an essential difference between it and the non-porous carbon surface, thus affecting its adsorption mechanism.
Physical adsorption first occurs in the micropores with the smallest size and the highest potential energy, and then gradually expands to the micropores with larger size and lower potential energy. The adsorption of micropores is not carried out layer by layer along the surface, but is achieved by solvent filling, while macropores and mesopores are surface adsorption mechanisms. Therefore, the adsorption performance of activated carbon mainly depends on its pore structure, especially the micropore structure, and the presence of a large number of mesopores also has a certain influence on adsorption.
Heycarbons Activated carbon physical form
The particle size of activated carbon also affects its adsorption performance. For example, the time it takes for the same amount of methylene blue to be adsorbed from a solution by the same activated carbon varies depending on its particle size. For example, the adsorption rate of activated carbon with a particle size of 325 mesh (diameter 0.043mm) is 375 times that of activated carbon with a particle size of 20 mesh (diameter 0.833mm) [i.e., equal to (0.833/0.043].
However, it cannot be assumed that the surface area of finely ground activated carbon is greater than that of the same amount of activated carbon with larger particles. Because the surface area exists in the vast, rich internal pore structure, grinding does not affect the surface area of the activated carbon, but it affects the time it takes to reach the equilibrium adsorption value.
Heycarbons Activated carbon surface chemical functional groups
The adsorption characteristics of activated carbon depend not only on its pore structure, but also on its surface chemical properties. The specific surface area and pore structure affect the adsorption capacity of activated carbon, while the surface chemical properties affect the interaction between activated carbon and polar or non-polar adsorbates.
The surface chemical properties of activated carbon are mainly determined by surface chemical functional groups, surface heteroatoms and compounds. Different surface functional groups, heteroatoms and compounds have obvious adsorption differences for different adsorbates. Generally speaking, the richer the acidic compounds in the surface functional groups, the more conducive to the adsorption of polar compounds, while alkaline compounds are conducive to the adsorption of weakly polar or non-polar substances.
Activated carbon treated with strong oxidants under appropriate conditions can increase the relative content of surface acidic groups, increase surface polarity, and thus enhance its adsorption capacity for polar compounds. Commonly used oxidants include HN, etc. Experimental studies have shown that after strong oxidation surface treatment of activated carbon, 11 different gases and steam were adsorbed.
The results show that the adsorption capacity of modified activated carbon for benzene, ethylamine, etc. is greatly reduced, mainly because the surface of activated carbon loses a large number of micropores after strong oxidation; while the adsorption capacity for ammonia and water is greatly enhanced, which is mainly due to the increase of oxides on the surface of activated carbon. Therefore, with the increase of oxides on the surface of activated carbon, its chemical adsorption of polar molecules is also enhanced.
By using a reducing agent to reduce the surface of activated carbon, the relative content of basic groups on the surface of activated carbon can be increased, the non-polarity of the surface can be increased, and the adsorption capacity of activated carbon for non-polar substances can be improved. Commonly used reducing agents are NaOH, NaOH, etc. Activated carbon after surface reduction exhibits different characteristics when treating dyes.
For anionic dyes, there is a close connection between the alkalinity of the activated carbon surface and the adsorption effect. The adsorption mechanism is the interaction between the oxygen-free Lewis base sites on the surface of activated carbon and the free electrons of the adsorbed dyes. For cationic dyes, the oxygen-containing functional groups on the surface of activated carbon play a positive role, but the heat-treated activated carbon still has a good adsorption effect on cationic dyes, which shows that electrostatic adsorption and dispersion adsorption are two equivalent adsorption mechanisms.
By liquid phase deposition, specific heteroatoms and compounds can be introduced on the surface of activated carbon, and the adsorption capacity of activated carbon can be increased by utilizing the binding effect between these substances and adsorbents. During liquid phase deposition, the type of impregnant is the main factor affecting the adsorption effect of activated carbon. Different impregnants can be used to treat activated carbon for different adsorbents to obtain good adsorption effects.
It is worth noting that when the surface functional groups of activated carbon are modified, the surface chemical properties of activated carbon will also change. Its surface area, pore volume and pore size distribution will change to a certain extent, which will also affect the adsorption of activated carbon. Therefore, when modifying the surface functional groups, different modifications should be adopted for different adsorption conditions and adsorbents, and the effects caused by the dual changes of physical structure and activated charcoal chemical structure should be comprehensively considered.
Heycarbons Activated carbon adsorbate
The adsorption effect of activated carbon is closely related to the properties of the adsorbate itself. Generally, when the “screening” effect of the activated carbon’s own pore structure on macromolecules is not considered, macromolecules are more easily adsorbed due to their higher adsorption energy.
For small molecular organic matter in water, those with large molecular weight are more easily adsorbed by activated carbon.For volatile organic compounds, the larger the molecular weight, the higher the removal rate, while for extractable organic matter, the opposite is true, and the adsorption effect increases as the molecular weight decreases. This is because the polarity of volatile organic compounds is relatively small, while the polarity of extractable organic compounds is relatively large.
Due to the nature of activated carbon itself, it can be regarded as a non-polar adsorbent, so it is easier to adsorb non-polar substances in water than polar substances. Moreover, when the molecular size of the adsorbate is in a certain ratio with the activated carbon, it is most conducive to adsorption.
Gases that are easily liquefied or have high boiling points are more easily adsorbed. In a mixed gas, the gas that is easily adsorbed in a pure state is adsorbed first. Generally, inorganic substances are not easily adsorbed, but molybdates, gold chloride, mercuric chloride, silver salts and iodized salts are exceptions.
Traube’s law states that the surface activity of an aqueous solution is proportional to the number of carbon atoms in the organic solute. According to Gibbs’ adsorption theory, the more a substance can reduce the surface tension of a solution, the easier it is to be adsorbed. Therefore, the order of the adsorption amount of alcohols can be obtained: methanol < ethanol < propanol < butanol < … , and the same is true for fats and aldehydes.
In the case of similar molecular weights, the presence of olefinic bond structure is conducive to the adsorption of activated carbon; straight-chain organic matter is easier to be adsorbed than branched organic matter. As the carbon chain grows, the adsorption amount of activated carbon also increases accordingly, acetic acid < propionic acid < butyric acid.
Heycarbons Activated carbon application conditions
The adsorption performance of activated carbon is not only directly related to the above factors, but also closely related to its application conditions.
Effect of temperature on adsorption capacity
At present, no satisfactory conclusion can be drawn theoretically for this item. According to the Langmuir hypothesis, adsorption is a dynamic equilibrium reaction. Changes in temperature increase the K value, indicating that the adsorption rate also increases and a new equilibrium is reached, thus changing the adsorption capacity of activated carbon.
The saturated adsorption capacity Xm means the adsorption capacity when the surface of the adsorbent is fully adsorbed by a single molecular layer, so Xm is a fixed value and is not affected by other factors. Generally, the adsorption process is an exothermic process, so an increase in temperature reduces the adsorption capacity and weakens the adsorption capacity. However, in the actual working system, the influence of temperature should be comprehensively considered according to different situations.
The effect of pressure on adsorption capacity
As the pressure increases, the gas adsorption capacity increases. Especially for gases with low adsorption capacity under normal pressure conditions, the increase in pressure has a positive effect on the adsorption performance, which is also the theoretical basis of pressure swing adsorption.
The influence of adsorbate concentration on adsorption capacity
In terms of the properties of the adsorbate, its solubility, molecular polarity, and molecular weight all have a certain influence on the adsorption performance. For the same substance, the adsorption capacity increases with the increase of adsorbate concentration at the beginning, forming a straight line, and then slowly increases.
After reaching a certain adsorption capacity, it will no longer change. The experimental data were processed using the Froderich formula and the Langmuir isotherm adsorption formula respectively. The result is basically a straight line, but the degree of fit between different organic substances and the straight line will vary.
Effect of pH value on adsorption capacity
The effect of pH value on different adsorbates is also different. For non-ionic adsorbates, the adsorption capacity has little to do with pH value; for cationic adsorbates, the adsorption capacity increases with the increase of pH value; for anionic adsorbates, the adsorption capacity decreases with the increase of pH value.
At the same time, the pH value of the solution also affects the adsorption of substances by oxygen-containing functional groups on the surface of activated carbon.
Effect of packing density of activated carbon adsorbent on adsorption capacity
The influence of the packing density of activated carbon adsorbent on the adsorption capacity. It is generally believed that a high packing density is more conducive to the adsorption of activated carbon.
When using activated carbon, it is necessary to comprehensively consider the specific experimental equipment and experimental conditions, weigh the influence of these factors, and find the best experimental application conditions.
Chapter 6
Heycarb Activated Carbon Selecting
Based on Application
Heycarbons Activated Carbon Selecting Based on Application
Activated carbon in water treatment and for wastewater treatment
Activated carbon air and gas purification
Activated carbon air and gas purification
Activated carbon food and beverage industry
Activated carbon for medicine and health
Activated carbon for environmental protection
Activated carbon selection criteria

How to choose the appropriate activated carbon according to its application?
Choosing the right activated carbon requires considering its specific purpose, physical and chemical properties, and the environment in which it is used. The following are some common activated carbon selection guidelines based on its different application areas:
Heycarbons Activated carbon in water treatment and for wastewater treatment
- Removal of organic pollutants: Choose granular activated carbon (GAC) with high adsorption capacity. Its large surface area and pore structure are suitable for the removal of organic compounds, pesticides, dissolved organic matter, etc.
- Removal of chlorine and byproducts: Use powdered activated carbon (PAC) because of its small particle size and rapid adsorption capacity to effectively remove chlorine and chlorination byproducts.
- Removal of heavy metals: Specially modified or impregnated activated carbon can be used to remove heavy metal ions such as copper, lead and cadmium from water.
learn more: Activated carbon for water treatment,Wastewater treatment,Water filter
https://heycarbons.com/activated-carbon-for-water-treatment-application/
Heycarbons Activated carbon in air and gas purification
- Removal of harmful gases: Selecting activated carbon impregnated with specific chemicals can effectively remove harmful gases such as ammonia and hydrogen sulfide.
- Removal of volatile organic compounds (VOCs): Use activated carbon fibers or granular activated carbon with high specific surface area, because they can quickly adsorb and fix VOCs.
- Flue gas purification: Granular activated carbon is usually used, and products with specific pore sizes are selected according to needs to effectively remove harmful substances in flue gas.
learn more:Activated Carbon For Air Purification,VOCs,Gases
Activated Carbon For Air Purification Application
Heycarbons Activated carbon in food and beverage industry
- Decolorization: Use high-porosity powdered activated carbon, which is suitable for the decolorization process of syrup, juice and other products.
- Deodorization: Choose activated carbon with rich microporous structure to effectively remove odor and bad smell.
Heycarbons Activated carbon for medicine and health
- Detoxification: Medical activated carbon, usually in ultra-pure granular or powdered form, is used to absorb toxins and drug overdoses.
- Air purification: Activated carbon used in medical environments needs to have the ability to efficiently remove pathogens and volatile organic compounds.
Heycarbons Activated carbon for industrial applications
- Solvent recovery: Selecting activated carbon with larger pore size is conducive to the adsorption and recovery of macromolecular solvents.
- Metal recovery: For example, in the process of gold smelting, select appropriate activated carbon for the adsorption and recovery of gold.
Heycarbons Activated carbon for environmental protection
- Soil remediation: Granular activated carbon is used to adsorb and fix organic pollutants in the soil to prevent them from spreading.
- Wastewater treatment: According to the type and concentration of pollutants in the wastewater, select appropriate granular activated carbon or powdered activated carbon to enhance the wastewater treatment effect.
Heycarbons Activated carbon selection criteria
- Specific surface area: The larger the specific surface area, the stronger the adsorption capacity. Suitable for occasions where high-efficiency adsorption is required.
- Pore size distribution: The distribution of micropores, mesopores and macropores determines the adsorption capacity of activated carbon for different molecular sizes.
- Mechanical strength: Activated carbon with high mechanical strength is suitable for systems with high fluidity, reducing pulverization and wear.
- Purity and chemical stability: Medical or food grade activated carbon requires high purity and no harmful chemicals.
Summarize
Choosing the right activated carbon requires comprehensive consideration of its purpose, physical and chemical properties, and the specific needs of the environment in which it is used. By analyzing the specific application scenario in detail, the most suitable type of activated carbon can be selected to achieve the best use effect.